Trandolapril
Synonym(s):(2S,3aR,7aS)-1-[(2S)-2-[[(1S)-1-(Ethoxycarbonyl)-3-phenylpropyl]amino]-1-oxopropyl]octahydro-1H-indole-2-carboxylic acid;Mavik;Trandolapril
- CAS NO.:87679-37-6
- Empirical Formula: C24H34N2O5
- Molecular Weight: 430.54
- MDL number: MFCD00865776
- EINECS: 618-046-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
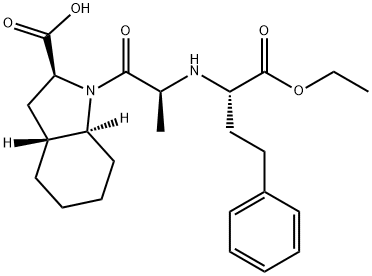
What is Trandolapril?
Absorption
~ 40-60% absorbed; extensive first pass metabolism results in a low bioavailability of 4-14%
Toxicity
Most likely clinical manifestations of overdose are symptoms of severe hypotension. Most common adverse effects include cough, headache and dizziness. The oral LD50 of trandolapril in mice was 4875 mg/kg in males and 3990 mg/kg in females. In rats, an oral dose of 5000 mg/kg caused low mortality (1 male out of 5; 0 females). In dogs, an oral dose of 1000 mg/kg did not cause mortality and abnormal clinical signs were not observed.
Description
Trandolapril is a new ACE inhibitor that is rapidly hydrolyzed, mainly in the liver, to its biologically active form, trandolaprilat. Compared with all other ACE inhibitors, trandolaprilat is reported to have the highest lipophilicity and the most prolonged ACE inhibitory activity. In hypertensive patients, trandolapril at a dose of 2 mg reduces blood pressure consistently throughout the 24 hour period after intake, making it one of the best once a day antihypertensive drugs. It has also been demonstrated to inhibit aortic atherosclerosis in the hyperlipidemic rabbit.
Chemical properties
White or almost white powder.
Originator
Roussel Uclaf (France)
The Uses of Trandolapril
Trandolapril has been used as an angiotensin-converting enzyme (ACE) inhibitor to study its effects on responsiveness of human retinal endothelial cells (HRECs) to vascular endothelial growth factor (VEGF).
The Uses of Trandolapril
An antihypertensive. Angiotensin converting enzyme (ACE) inhibitor
The Uses of Trandolapril
antibacterial
Background
Trandolapril is a non-sulhydryl prodrug that belongs to the angiotensin-converting enzyme (ACE) inhibitor class of medications. It is metabolized to its biologically active diacid form, trandolaprilat, in the liver. Trandolaprilat inhibits ACE, the enzyme responsible for the conversion of angiotensin I (ATI) to angiotensin II (ATII). ATII regulates blood pressure and is a key component of the renin-angiotensin-aldosterone system (RAAS). Trandolapril may be used to treat mild to moderate hypertension, to improve survival following myocardial infarction in clinically stable patients with left ventricular dysfunction, as an adjunct treatment for congestive heart failure, and to slow the rate of progression of renal disease in hypertensive individuals with diabetes mellitus and microalbuminuria or overt nephropathy.
Indications
For the treatment of mild to moderate hypertension, as an adjunct in the treatment of congestive heart failure (CHF), to improve survival following myocardial infarction (MI) in individuals who are hemodynamically stable and demonstrate symptoms of left ventricular systolic dysfunction or signs of CHF within a few days following acute MI, and to slow progression of renal disease in hypertensive patients with diabetes mellitus and microalbuminuria or overt nephropathy.
What are the applications of Application
Trandolapril is an angiotensin-converting enzyme (ACE) inhibitor
Definition
ChEBI: Trandolapril is a heterobicylic compound that is (2S,3aR,7aS)-1-[(2S)-2-aminopropanoyl]octahydro-1H-indole-2-carboxylic acid in which the hydrogen of the amino group is substituted by a (2R)-1-ethoxy-1-oxo-4-phenylbutan-2-yl group. It is a angiotensin-converting enzyme inhibitor and a prodrug used for the treatment of hypertension. It has a role as a prodrug, an antihypertensive agent and an EC 3.4.15.1 (peptidyl-dipeptidase A) inhibitor. It is a dicarboxylic acid monoester, a dipeptide, an ethyl ester, a secondary amino compound, a tertiary carboxamide and an organic heterobicyclic compound. It is functionally related to a trandolaprilat.
brand name
Odrik; Udrik; Gopten
General Description
Trandolapril, 1-[2-(1-ethoxycarbonyl-3-phenylpropylamino)propionyl]octahydroindole-2-carboxylicacid (Mavik), is an indole-containing ACE inhibitorthat is structurally related to most of the precedingagents discussed. Enalapril is very similar to trandolapril,with the primary difference occurring in the heterocyclicsystems. The pyrrolidine of enalapril has been replacedwith an octahydroindole system. Much like enalaprilate,trandolapril must be hydrolyzed to tranolaprilate, which isthe bioactive species.
Biochem/physiol Actions
Trandolapril is an ACE inhibitor. Trandolapril differs from other ACE inhibitors in that it has a longer biological half-life and a high degree of lipophilicity.
Pharmacokinetics
Trandolapril is the ethyl ester prodrug of a nonsulfhydryl ACE inhibitor, trandolaprilat. Trandolapril is deesterified in the liver to the diacid metabolite, trandolaprilat, which is approximately eight times more active as an inhibitor of ACE than its parent compound. ACE is a peptidyl dipeptidase that is part of the RAAS. The RAAS is a homeostatic mechanism for regulating hemodynamics, water and electrolyte balance. During sympathetic stimulation or when renal blood pressure or blood flow is reduced, renin is released from the granular cells of the juxtaglomerular apparatus in the kidneys. In the blood stream, renin cleaves circulating angiotensinogen to ATI, which is subsequently cleaved to ATII by ACE. ATII increases blood pressure via a number of mechanisms. First, it stimulates the secretion of aldosterone from the adrenal cortex. Aldosterone travels to the distal convoluted tubule (DCT) and collecting tubule of nephrons where it increases sodium and water reabsorption by increasing the number of sodium channels and sodium-potassium ATPases on cell membranes. Second, ATII stimulates the secretion of vasopressin (also known as antidiuretic hormone or ADH) from the posterior pituitary gland. ADH stimulates further water reabsorption from the kidneys via insertion of aquaporin-2 channels on the apical surface of cells of the DCT and collecting tubules. Third, ATII increases blood pressure through direct arterial vasoconstriction. Stimulation of the Type 1 ATII receptor on vascular smooth muscle cells leads to a cascade of events resulting in myocyte contraction and vasoconstriction. In addition to these major effects, ATII induces the thirst response via stimulation of hypothalamic neurons. ACE inhibitors inhibit the rapid conversion of ATI to ATII and antagonize RAAS-induced increases in blood pressure. ACE (also known as kininase II) is also involved in the enzymatic deactivation of bradykinin, a vasodilator. Inhibiting the deactivation of bradykinin increases bradykinin levels and may further sustain the effects of trandolaprilat by causing increased vasodilation and decreased blood pressure. The blood pressure lowering effect of trandolaprilat is due to a decrease in peripheral vascular resistance, which is not accompanied by significant changes in urinary excretion of chloride or potassium or water or sodium retention.
Clinical Use
Angiotensin converting enzyme inhibitor:
Hypertension
Heat failure
After myocardial infarction
Drug interactions
Potentially hazardous interactions with other drugs
Anaesthetics: enhanced hypotensive effect.
Analgesics: antagonism of hypotensive effect and
increased risk of renal impairment with NSAIDs;
hyperkalaemia with ketorolac and other NSAIDs.
Antihypertensives: increased risk of hyperkalaemia,
hypotension and renal failure with ARBs and
aliskiren.
Bee venom extract: possible severe anaphylactoid
reactions when used together.
Ciclosporin: increased risk of hyperkalaemia and
nephrotoxicity.
Cytotoxics: increased risk of angioedema with
everolimus.
Diuretics: enhanced hypotensive effect;
hyperkalaemia with potassium-sparing diuretics.
ESAs: increased risk of hyperkalaemia; antagonism
of hypotensive effect.
Gold: flushing and hypotension with sodium
aurothiomalate.
Lithium: reduced excretion (possibility of enhanced
lithium toxicity).
Potassium salts: increased risk of hyperkalaemia.
Tacrolimus: increased risk of hyperkalaemia and
nephrotoxicity.
Metabolism
Cleavage of the ester group of trandolapril, primarily in the liver, is responsible for conversion to trandolaprilat, the active metabolite. Seven other metabolites, including diketopiperazine and glucuronide conjugated derivatives of trandolapril and trandolaprilat, have been identified.
Metabolism
Trandolapril is metabolised in the liver to the active
trandolaprilat and to some inactive metabolites. About
33
% of an oral dose of trandolapril is excreted in the
urine, mainly as trandolaprilat; the rest is excreted in the
faeces.
Properties of Trandolapril
| Melting point: | 122-123°C |
| Boiling point: | 626.0±55.0 °C(Predicted) |
| Density | 1.181±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: ≥20mg/mL |
| form | white powder |
| pka | 3.15±0.20(Predicted) |
| color | White to Off-White |
| CAS DataBase Reference | 87679-37-6(CAS DataBase Reference) |
Safety information for Trandolapril
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Trandolapril
Trandolapril manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 87679-37-6 Trandolapril 99%View Details
87679-37-6 Trandolapril 99%View Details
87679-37-6 -
 87679-37-6 98%View Details
87679-37-6 98%View Details
87679-37-6 -
 Trandolapril 98%View Details
Trandolapril 98%View Details
87679-37-6 -
 Trandolapril CAS 87679-37-6View Details
Trandolapril CAS 87679-37-6View Details
87679-37-6 -
 Trandolapril CAS 87679-37-6View Details
Trandolapril CAS 87679-37-6View Details
87679-37-6 -
 Trandolapril CAS 87679-37-6View Details
Trandolapril CAS 87679-37-6View Details
87679-37-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1