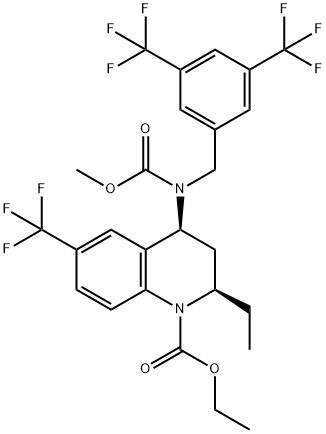
Torcetrapib
Synonym(s):(2R,4S)-4-((3,5-Bis-trifluoromethylbenzyl)methoxycarbonylamino)-2-ethyl-6-trifluoromethyl-3,4-dihydro-2H-quinoline-1-carboxylic acid ethyl ester;CP-529,414;CP-529414
- CAS NO.:262352-17-0
- Empirical Formula: C26H25F9N2O4
- Molecular Weight: 600.47
- MDL number: MFCD09260777
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
What is Torcetrapib?
Chemical properties
Off-White Low Melting Solid
The Uses of Torcetrapib
Cholesteryl ester transfer protein (CETP) inhibitor. Antilipemic; antiatherosclerotic.
Definition
ChEBI: Torcetrapib is a member of quinolines, a carbamate ester and a member of (trifluoromethyl)benzenes. It has a role as an anticholesteremic drug and a CETP inhibitor.
Biological Activity
torcetrapib is a cetp inhibitor with ic50 of 37 nm, elevates hdl-c and reduces nonhdl-c in plasma. inhibition of cholesteryl ester transfer protein (cetp) has been shown to have a substantial effect on plasma lipoprotein levels.
in vitro
torcetrapib dose-dependently increases aldosterone release from h295r cells after either 24 or 48 h of treatment, this effect is mediated by calcium channel as calcium channel blockers completely blocks torcetrapib-induced corticoid release and calcium increase. torcetrapib (1 μm) significantly increases the expression of steroidogenic gene, cyp11b2 and cyp11b1, in h295r cell lines [1].
in vivo
researchers tested torcetrapib in rabbits fed an atherogenic diet at a dose sufficient to increase hdl-c by at least 3-fold. cetp activity was inhibited by 70–80% throughout the study. non-hdl-c increased in both groups, but there was no difference apparent by the study’s end [2].
storage
Room temperature
References
[1] hu x, dietz jd, xia c, knight dr, loging wt, smith ah, yuan h, perry da, keiser j. torcetrapib induces aldosterone and cortisol production by an intracellular calcium-mediated mechanism independently of cholesteryl ester transfer protein inhibition. endocrinology. 2009;150(5):2211-9.
[2] morehouse la, sugarman ed, bourassa pa, sand tm, zimetti f, gao f, rothblat gh, milici aj. inhibition of cetp activity by torcetrapib reduces susceptibility to diet-induced atherosclerosis in new zealand white rabbits. j lipid res. 2007;48(6):1263-72.
[3] barter pj, caulfield m, eriksson m, grundy sm, kastelein jj, komajda m, lopez-sendon j, mosca l, tardif jc, waters dd, shear cl, revkin jh, buhr ka, fisher mr, tall ar, brewer b; illuminate investigators. effects of torcetrapib in patients at high risk for coronary events. n engl j med. 2007;357(21):2109-22.
Properties of Torcetrapib
| Melting point: | 54-58°C |
| Boiling point: | 504.8±50.0 °C(Predicted) |
| Density | 1.42 |
| storage temp. | Store at RT |
| solubility | DMSO: >5mg/mL |
| form | powder |
| pka | -1.87±0.40(Predicted) |
| color | white |
| optical activity | [α]/D >-70° |
| CAS DataBase Reference | 262352-17-0(CAS DataBase Reference) |
Safety information for Torcetrapib
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for Torcetrapib
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 Torcetrapib 98% (HPLC) CAS 262352-17-0View Details
Torcetrapib 98% (HPLC) CAS 262352-17-0View Details
262352-17-0 -
 Torcetrapib CAS 262352-17-0View Details
Torcetrapib CAS 262352-17-0View Details
262352-17-0 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1