Topiroxostat
- CAS NO.:577778-58-6
- Empirical Formula: C13H8N6
- Molecular Weight: 248.24
- MDL number: MFCD17167057
- EINECS: 1592732-453-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-22 18:23:36
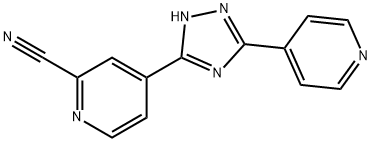
What is Topiroxostat?
Description
Topiroxostat is an orally-administered, non-purine, selective xanthine oxidase (XO) inhibitor developed for the treatment of hyperuricemia specifically for patients with gout in Japan. The drug was discovered and developed by Fuji Yakuhin. In contrast to conventional XO inhibitors such as febuxostat, topiroxostat interacts with key amino acid residues of the solvent channel.
Chemical properties
Topiroxostat is soluble in organic solvents such as DMSO and dimethyl formamide (DMF). The solubility of topiroxostat in these solvents is approximately 10 and 20 mg/ml, respectively.
The Uses of Topiroxostat
Topiroxostat is a selective xanthine oxidase inhibitor developed for treatment and management of hyperuricemia and gout. Xanthine oxidase, or xanthine oxidoreductase (XOR), regulates purine metabolism, and inhibition of the enzyme results in efficacious reduction of serum urate levels. Approved for therapeutic use in Japan since 2013, topiroxostat is marketed under the name Topiloric and Uriadec and is orally administered twice daily.
Biological Activity
Topiroxostat is an inhibitor of xanthine oxidoreductase (IC50 = 5.3 nM), an enzyme that converts xanthine to urate. Formulations containing topiroxostat are effective in vivo, lowering urate levels in the serum of patients with hyperuricemia. In rats, but not primates, topiroxostat treatment results in the formation of xanthine crystals, resulting in nephritis. Topiroxostat also inhibits ATP-binding cassette transporter G2 (ABCG2; IC50 = 0.18 μM), a high-capacity urate transporter that functions in both renal and extrarenal urate secretion.
Pharmacology
Topiroxostat is a novel inhibitor of xanthine oxidase, and is postulated to exert a renoprotective effect. Puromycin aminonucleoside nephrosis (PAN) is a rat model of minimal change nephrotic syndrome. In this study, we examined whether topiroxostat ameliorates the kidney injury in PAN rats that was induced by a single intraperitoneal injection of PA (100mg/kg body weight). Rats were divided into four groups: control rats, PAN rats, control rats treated with topiroxostat (1.0mg/kg/day), and PAN rats treated with topiroxostat. Topiroxostat significantly reduced the amount of uric acid in the kidney cortex, while serum UA concentration remained unaffected by this treatment. Urinary protein excretion decreased significantly on day 10 in PAN rats upon topiroxostat treatment. Podocyte injury in PAN rats, as indicated by the reduction in WT‐1‐positive cell numbers and podocin immunoreactivity and foot process effacement, was partially yet significantly alleviated with topiroxostat treatment. In the kidney cortex, the increase in oxidative stress markers such as nitrotyrosine and 8‐hydroxy‐2‐deoxyguanosine (8‐OHdG) and the enhanced expressions of xanthine oxidase and NADPH oxidase 4 (NOX4) in PAN rats were significantly ameliorated by topiroxostat. Using cultured podocytes NOX4 expression was upregulated by adding 12mg/dL UA into the culture medium. These results suggest that topiroxostat ameliorates proteinuria and kidney injury in PAN rats by lowering oxidative stress and tissue UA concentration. The renoprotective effects of topiroxostat could be attributed to its potential to inhibit xanthine oxidase and NOX4 in concert with suppression of intracellular UA production. Abstract : Topiroxostat ameliorates proteinuria and renal injury independently of serum UA level in PAN‐induced nephrotic rats. Topiroxostat exerts a renoprotective effect owing to its antioxidant effects and property to lower UA levels in the kidney cortex.
Clinical Use
Topiroxostat, a novel XO inhibitor, ameliorated proteinuria in association with podocyte injury markers such as decrease in WT-1 and podocin expressions and foot process effacement on electron microscope.
Synthesis
The synthesis commenced with the reaction of two commercially available components, nitrile oxide 177 and isonicotinohydrazide (178). Condensation of these two subunits in the presence of sodium methoxide followed by acidic quench gave rise to 1,2,4-triazole 179 in 89% yield. Next, utilization of the N-oxide for installation of the nitrile functionality was required to furnish the drug, but it is interesting to note that this step has been the subject of study by Yamamoto and co-workers at Tohoku University in Japan. Although the process preparation describes the formation of the drug using sodium cyanide and dimethylcarbamoyl chloride followed by isolation through a salt formation/ freebasing process to deliver topiroxostat (XXIII) in 66% yield, Yamamoto has described this same sequence using zinc cyanide and tosylate salt formation (freebasing of the drug was not attempted).
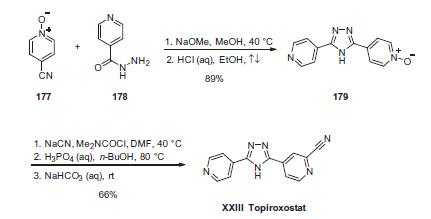
Carcinogenicity
Carcinogenicity was evaluated in 2-year oral dose studies in mice and rats. Tumors developed in both animal species (mice, mammary adenocarcinoma; rats, transitional cell papilloma/papillary carcinoma in the kidney and bladder, transitional cell carcinoma in the ureter, renal cell carcinoma and angiosarcoma in the kidney, follicular cell adenoma in the thyroid), secondary to chronic renal impairment probably due to xanthine crystal deposition. Comparison of the exposure (AUC)7 to unchanged topiroxostat at the non-carcinogenic doses in mice and rats and at the maximum recommended clinical dose yielded a safety margin of <1-fold in both animal species. However, the applicant has considered that the observed tumorigenesis is not relevant to humans because xanthine crystal deposition is pronounced in rodents in comparison with other animal species including humans.
Properties of Topiroxostat
| Boiling point: | 594.7±60.0 °C(Predicted) |
| Density | 1.45±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | DMSO : 23.5 mg/mL (94.67 mM; Need ultrasonic and warming) |
| form | Powder |
| pka | 7.47±0.10(Predicted) |
| color | White to off-white |
| InChI | InChI=1S/C13H8N6/c14-8-11-7-10(3-6-16-11)13-17-12(18-19-13)9-1-4-15-5-2-9/h1-7H,(H,17,18,19) |
Safety information for Topiroxostat
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Topiroxostat
| InChIKey | UBVZQGOVTLIHLH-UHFFFAOYSA-N |
| SMILES | C1(C#N)=NC=CC(C2NN=C(C3C=CN=CC=3)N=2)=C1 |
Topiroxostat manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 577778-58-6 Topiroxostat 99%View Details
577778-58-6 Topiroxostat 99%View Details
577778-58-6 -
 Topiroxostat CAS 577778-58-6View Details
Topiroxostat CAS 577778-58-6View Details
577778-58-6 -
 Topiroxo Topiroxostat 40mg Tablet - TopiloricView Details
Topiroxo Topiroxostat 40mg Tablet - TopiloricView Details
577778-58-6 -
 Topiroxostat CAS Number:577778-58-6View Details
Topiroxostat CAS Number:577778-58-6View Details
577778-58-6 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
