Titanocene dichloride
Synonym(s):Cp2TiCl2;Titanocene dichloride;Dichlorotitanocene;Di(cyclopentadienyl)titanium(IV) dichloride;Dichlorobis(1,3-cyclopentadiene)titanium
- CAS NO.:1271-19-8
- Empirical Formula: C10H10Cl2Ti
- Molecular Weight: 248.96
- MDL number: MFCD00003723
- EINECS: 215-035-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-02-23 21:28:46
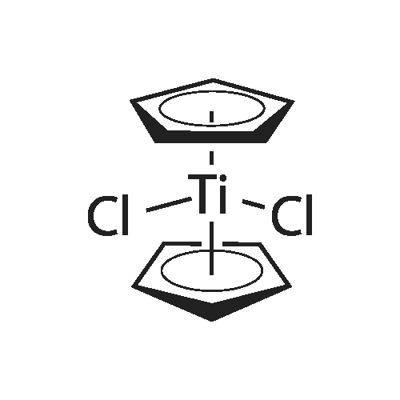
What is Titanocene dichloride?
Description
Titanocene dichloride is an organotitanium compound with the chemical formula (η5-C5H5)2TiCl2, often written as Cp2TiCl2, which is a common reagent in organometallic chemistry and organic synthesis. Cp2TiCl2 does not form a "sandwich" structure like ferrocene, but a tetrahedral structure due to its four ligands around a metal center. Because of its anti-tumor activity, it has been used in clinical trials as a chemotherapeutic agent.
Chemical properties
Titanocene dichloride is a reddish-orange crystalline solid. Moderately soluble in toluene, chloroform, alcohol, and other hydroxylic solvents; sparingly soluble in water, petroleum ether, benzene, ether, carbon disulfide, and carbon tetrachloride. Stable in dry air, slowly hydrolyzed in moist air. Titanocene dichloride is irritating to the skin and mucous membranes.
The Uses of Titanocene dichloride
Titanocene dichloride is used as an experimental cancer chemotherapeutic agent. It is used as a research chemical, as a catalyst in Ziegler–Natta polymerization reactions, and as an implant material in orthopedics, oral surgery, and neurosurgery.This metallocene is a common reagent in organometallic and organic synthesis. Titanocene dichloride is used to prepare titanocene pentasulfide. Used as an anticancer drug.
What are the applications of Application
Bis(cyclopentadienyl)titanium(IV) dichloride is a reagent in organometallic and organic synthesis
Production Methods
Titanocene dichloride is produced by the reaction of titanium tetrachloride with cyclopentadienyl sodium.
Preparation
Cp2TiCl2 continues to be prepared similarly to its original synthesis by Wilkinson and Birmingham:
2 NaC5H5 + TiCl4 → (C5H5)2TiCl2 + 2 NaCl
The reaction is conducted in THF. Work-up entails extraction into chloroform/hydrogen chloride and recrystallization from toluene. In the original literature, the structure was poorly understood. Each of the two Cp rings are attached to Ti(IV) through all five carbon atoms. In organometallic chemical jargon, this bonding is referred to as η5 (see hapticity).
Reactions
- Precatalyst for reduction of esters and |á,|?-unsaturated ketones.
- Catalyzes reductive deoxygenation of alcohols and hydroxylamines.
- Catalyst for the radical cyclization of epoxides.
- Reagent for the conversion of enynes to bicyclic cyclopentenones.
- Catalyzes silylation of alkenes and alkynes.


General Description
Titanocene dichloride appears as red to red-orange crystals. (NTP, 1992)
Air & Water Reactions
Stable in dry air. Decomposes in moist air and in water to form HCl .
Reactivity Profile
Titanocene dichloride is incompatible with strong oxidizers. Titanocene dichloride may decompose on exposure to water. .
Hazard
Toxic by inhalation, irritant to skin and mucous membranes.
Fire Hazard
Flash point data for Titanocene dichloride are not available; however, Titanocene dichloride is probably combustible.
Flammability and Explosibility
Non flammable
Safety Profile
Poison by intravenous and intraperitoneal routes. Questionable carcinogen with experimental neoplastigenic, tumorigenic, and teratogenic data. Mutation data reported. See also TITANIUM COMPOUNDS. When heated to decomposition it emits toxic fumes of Cl-
Carcinogenicity
Based on the results of 2 year gavage studies, the National Toxicology Program determined that there was equivocal evidence of the carcinogenic activity of titanocene dichloride in male and female rats based on a marginal increase in the incidence of forestomach squamous cell effects.
Purification Methods
It forms bright red crystals from toluene or xylene/CHCl3 (1:1) and sublimes at 190o/2mm. It is moderately soluble in EtOH and insoluble in Et2O, *C6H6, CS2, CCl4, pet ether and H2O. The crystalline dipicrate explodes on melting at 139-140o. [Wilkinson et al. J Am Chem Soc 75 1011 1953, IR: Wilkinson & Birmingham J Am Chem Soc 76 4281 1954, NMR and X-ray: Glivicky & McCowan Can J Chem 51 2609 1973, Clearfield et al. J Am Chem Soc 53 1622 1975, Beilstein 16 IV 1769.]
Clinical claims and research
Titanocene dichloride, [Ti(Z5 -C5H5)2Cl2], was the first organometallic transition metal compound to be investigated clinically as an anticancer agent. It contains a cis-dichloride motif as cisplatin and was selected from several early transition metal cyclopentadienyl complexes as the best candidate for further development. The chemistry of the hard Ti(IV) ion is different from that of Pt(II): for example, cisplatin binds preferentially to the N7 of guanine in DNA, whereas titanocene dichloride exhibits higher affinity for the phosphate backbone. [Ti(Z5 -C5H5)2Cl2] hydrolyzes quickly in water, yielding a solvated Ti(IV) ion with high affinity for transferrin. As for Ga(III), selective transport of titanium ions via the transferrin route appears plausible. In vitro, titanocene dichloride is active in cisplatin-resistant cancer cells. It entered clinical trials in 1993, revealing nephrotoxicity as dose-limiting side effect. In Phase II studies as singleagent therapy no advantage over other treatment regimens was observed, and the trials were thus abandoned.
Properties of Titanocene dichloride
| Melting point: | 260-280 °C (dec.)(lit.) |
| Boiling point: | 355.52°C (estimate) |
| Density | 1.6 g/mL at 25 °C(lit.) |
| vapor pressure | 0.002Pa at 25℃ |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | slightly soluble in H2O, benzene; soluble in chloroform,ethanol, toluene |
| form | crystal |
| Specific Gravity | 1.6 |
| color | red |
| Water Solubility | slow decomposition |
| Sensitive | Air & Moisture Sensitive |
| Hydrolytic Sensitivity | 4: no reaction with water under neutral conditions |
| Merck | 14,9482 |
| Stability: | Stable. Incompatible with strong oxidizing agents. Decomposes in water. Moisture sensitive. |
| CAS DataBase Reference | 1271-19-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Titanocene dichloride(1271-19-8) |
| EPA Substance Registry System | Titanocene dichloride (1271-19-8) |
Safety information for Titanocene dichloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Titanocene dichloride
Titanocene dichloride manufacturer
JSK Chemicals
New Products
Tetrabutylammonium iodide (3,3-DIFLUOROCYCLOBUTYL)METHANOL 4,4-DIFLUOROCYCLOHEXANAMINE Cyclobutylamine (S)-3-Fluoro-pyrrolidine hydrochloride 3-Oxocyclobutanecarboxylic acid N-Hydroxy-2-methylpropanimidamide L-tert-Leucine,97% 2-Bromophenylacetonitrile, 97% Aluminum oxide, basic Calcium hydroxide, 95% Diallylamine, 99% 2-Iodobenzoic Acid 3-Methoxybenzonitrile Pentachlorobenzonitrile Chloral Dibenzoyl Peroxide Titanium Dioxide O-Benzylhydroxylamine Hydrochloride 2-Nitrobenzaldehyde 2-Picolylamine (2-Aminomethylpyridine) 2-Venylpyridine Ethyl-2-Chloroacetoacetate 4-Dimethylamine PyridineRelated products of tetrahydrofuran

You may like
-
 Bis(cyclopentadienyl)titanium dichloride CAS 1271-19-8View Details
Bis(cyclopentadienyl)titanium dichloride CAS 1271-19-8View Details
1271-19-8 -
 Bis(cyclopentadienyl)titanium dichloride CAS 1271-19-8View Details
Bis(cyclopentadienyl)titanium dichloride CAS 1271-19-8View Details
1271-19-8 -
 Bis(cyclopentadienyl)titanium dichloride CAS 1271-19-8View Details
Bis(cyclopentadienyl)titanium dichloride CAS 1271-19-8View Details
1271-19-8 -
 Titanocene dichloride 97% CAS 1271-19-8View Details
Titanocene dichloride 97% CAS 1271-19-8View Details
1271-19-8 -
 Titanocene Dichloride CAS 1271-19-8View Details
Titanocene Dichloride CAS 1271-19-8View Details
1271-19-8 -
 1271-19-8 Titanocene dichloride, 98% 99%View Details
1271-19-8 Titanocene dichloride, 98% 99%View Details
1271-19-8 -
 Titanocene dichloride 98% (HPLC) CAS 1271-19-8View Details
Titanocene dichloride 98% (HPLC) CAS 1271-19-8View Details
1271-19-8 -
 Bis(cyclopentadienyl)titanium(IV) dichloride CAS 1271-19-8View Details
Bis(cyclopentadienyl)titanium(IV) dichloride CAS 1271-19-8View Details
1271-19-8
