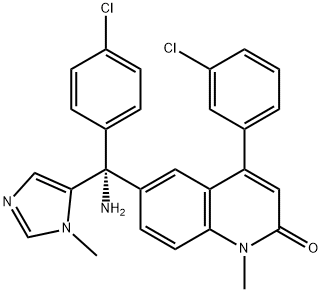
Tipifarnib
Synonym(s):(R)-(+)-R 115777;6-[(R)-Amino(4-chlorophenyl)(1-methyl-1H-imidazol-5-yl)methyl]-4-(3-chlorophenyl)-1-methyl-2(1H)-quinolinone;R115777
- CAS NO.:192185-72-1
- Empirical Formula: C27H22Cl2N4O
- Molecular Weight: 489.4
- MDL number: MFCD07772347
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
What is Tipifarnib?
Chemical properties
Off-White to Pale Beige Solid
The Uses of Tipifarnib
A farnesyltransferase inhibitor. Sensitizes human multiple myeloma cell to proteasome inhibition by blocking degradation of bortezomib(B675700)-induced aggresomes. Also shown to inhibit the growth of myeloid leukemia cell lines and primary leukemia cells by inducing apoptosis and cell-cycle blockage when combined with rapamycin(R124000).
The Uses of Tipifarnib
A farnesyltransferase inhibitor. Sensitizes human multiple myeloma cell to proteasome inhibition by blocking degradation of bortezomib(B675700)-induced aggresomes. Also shown to inhibit the growth of myeloid leukemia cell lines and primary leukemia cells by inducing apoptosis and cell-cycle blockage when combined with rapamycin(R124000).
What are the applications of Application
Tipifarnib is an inhibitor of the kappa B-Ras peptide
Definition
ChEBI: Tipifarnib is a quinolone that is 1-methylquinolin-2-one which carries a 3-chlorophenyl and an amino(4-chlorophenyl)(1-methyl-imidazol-5-yl)methyl groups at the 4 and 6 positions, respectively (the R-isomer). It has a role as an antineoplastic agent, an EC 2.5.1.58 (protein farnesyltransferase) inhibitor and an apoptosis inducer. It is a quinolone, a member of monochlorobenzenes, a member of imidazoles and a primary amino compound.
General Description
Pre-clinical studies show that tipifarnib has antiproliferative effects on pancreatic cancer cell lines at clinically relevant concentrations (concentration that inhibits 50% growth [IC50] from 9.5 to 500?nmol/L). It also exhibits marked growth retardation and antiangiogenic effects in a pancreatic cancer xenograft model.
Biological Activity
tipifarnib (also known as zarnestra or r115777), an orally bioavailable quinolone analog of imidazole heterocyclics, is a potent and specific nonpeptidomimetic competitive inhibitor of farnesyltransferase (ftase), an enezyme mediating post-translational farnesylation of multiple protein substrates involved in tumor cell proliferation. it has demonstrated inhibition of growth and proliferation of a broad range of human tumor models (either wild-type or mutated ras) via cytostatic rather than cytotoxic activity both in vitro and in vivo. it cell-type dependently induces apoptosis in some neoplastic cell lineages other than acute myeloid leukemia (aml), including multiple myeloma (mm) cell lines and mm cultures from patients.p.k. epling-burnett and thomas p. loughran jr. suppression of farnesyltransferase activity in acute myeloid leukemia and myelodysplastic syndrome: current understanding and recommended use of tipifarnib. expert opin investig drugs. 2010; 19(5): 689-698jean-pierre armand, alan k. burnett, johannes drach, jean-luc harousseau, bob lowenberg and jesus san miguel. the emerging role of targeted therapy for hematologic malignancies: update on bortezomib and tipifarnib. the oncologist 2007, 12:281-290elzbieta izbicka, david campos, gilbert carrizales and amita patnaik. biomarkers of anticancer activity of r115777 (tipifarnib, zarnestra) in human breast cancer models in vitro. anticancer research 2005; 25: 3215-3224
Biochem/physiol Actions
Tipifarnib (R115777) is an orally active and potent farnesyltransferase (FTase) inhibitor that exhibits potent anti-tumorigenic effects. Tipifarnib mechanism of action is not fully understand.
Properties of Tipifarnib
| Melting point: | 211-213°C (dec.) |
| Boiling point: | 681.7±55.0 °C(Predicted) |
| Density | 1.33±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | powder |
| pka | 7.20±0.10(Predicted) |
| color | white to beige |
| optical activity | [α]/D 17 to 24°, c = 1 in methanol |
Safety information for Tipifarnib
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Tipifarnib
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![FARNESENE (MIXTURE OF ISOMERS)[FOR PERFUMERY]](https://img.chemicalbook.in/)


You may like
-
 Tipifarnib CAS 192185-72-1View Details
Tipifarnib CAS 192185-72-1View Details
192185-72-1 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1