Tiagabine hydrochloride
Synonym(s):(-)-(R)-1-[4,4-bis(3-methyl-2-thienyl)-3-butenyl]nipecotic acid hydrochloride;(R)-(-)-1-[4,4-Bis(3-methyl-2-thienyl)-3-butenyl]-3-piperidinecarboxylic acid hydrochloride monohydrate;(3R)-1-[4,4-Bis(3-methyl-2-thienyl)-3-buten-1-yl]-3-piperidinecarboxylic acid hydrochloride;Tiagabine hydrochloride monohydrate
- CAS NO.:145821-59-6
- Empirical Formula: C20H26ClNO2S2
- Molecular Weight: 412.01
- MDL number: MFCD07369025
- EINECS: 200-659-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33

What is Tiagabine hydrochloride?
Description
Tiagabine (145821-59-6) is a clinically useful anticonvulsant.1?It is also used to treat anxiety/panic disorder2?and neuropathic pain3. Tiagabine inhibits GABA-reuptake (IC50?[3H]GABA uptake = 67 nM)?via?inhibiting GABA transporter 1(GAT1) blocking both neuronal and glial GABA re-uptake.4,5
Chemical properties
White to Off-White Solid
Originator
Gabitril,Cephalon, Inc.,USA
The Uses of Tiagabine hydrochloride
Tiagabine Hydrochloride is a GABA uptake inhibitor and an anticonvulsant (1,2,3). It is used to treat epilepsy, anxiety disorders, and panic disorder. Neuroprotective & Neuroresearch Product.
The Uses of Tiagabine hydrochloride
A GABA uptake inhibitor. Anticonvulsant.
The Uses of Tiagabine hydrochloride
Tiagabine hydrochloride was used to study serotoninergic transmission in G1 cells. It has also been used to study its anti-aging effects on the sensory neurons of C. elegans.
What are the applications of Application
Tiagabine Hydrochloride is an uptake inhibitor of GABA and also an anticonvulsant
Definition
ChEBI: A hydrochloride resulting from the reaction of equimolar amounts of tiagabine and hydrogen chloride. A GABA reuptake inhibitor, it is used for the treatment of epilepsy.
Manufacturing Process
A solution of 34 ml of n-butyl lithium in 30 ml of anhydrous ether was cooled
to -65°C under nitrogen and 5.3 ml of 3-methyl-2-bromothiophene in 10 ml
anhydrous ether was added dropwise over a period of 10 min. The reaction
mixture was stirred at -65°C for 1 h and 2.7 ml of ethyl 4-bromo-butyrate in
10 ml of anhydrous ether was added slowly. The reaction was stirred for 4 h
while the temperature raised to -20°C, 20 ml water was added, and the
mixture was stirred for 5 min after which the aqueous layer was removed. The
ether layer was washed with 20 ml of water, and the combined aqueous
phases were extracted with 50 ml of ether. The combined organic phases were
dried over anhydrous sodium sulfate, which after evaporation yielded 9 g of 1-
bromo-4,4-bis(3-methylthien-2-yl)but-3-ene as an oil.
This compound was without further purification used for coupling with ethyl
nipecotate.
A suspension of 5.0 g of 1-bromo-4,4-bis(3-methylthien-2-yl)but-3-ene, 3.4 g
of nipecotic acid ethyl ester and 3.3 g of potassium carbonate in 150 ml of dry
acetone was kept under reflux for 15 h. The reaction mixture was evaporated
and, after addition of 30 ml of water, the resulting solution was extracted
twice with 50 ml of ethyl acetate. The ethyl acetate extracts were dried and
evaporated leaving 7.3 g of an oil. By column chromatography on silica gel
using methanol as eluent, N-(4,4-bis(3-methylthien-2-yl)but-3-enyl)nipecotic
acid ethyl ester was isolated.
5.3 g of N-(4,4-bis(3-methylthien-2-yl)but-3-enyl)nipecotic acid ethyl ester
was dissolved in 100 ml of ethanol and 200 ml of an 8 N sodium hydroxide
solution was added. The mixture was heated at reflux for 1 h, cooled and
acidified by adding 10% hydrochloric acid. The resulting solution was
evaporated and 100 ml of water was added to the residue. The resulting acid
solution was extracted with ethyl acetate and the dried extract was
evaporated to give (R)-N-(4,4-bis(3-methylthien-2-yl)but-3-enyl)nipecotic acid
hydrochloride, melting point 187°-189°C.
Therapeutic Function
Antiepileptic
Biochem/physiol Actions
Tiagabine increases the synaptic GABA and enhances the neuronal inhibition. It is effective as adjunctive treatment of epilepsy and anxiety disorders.
storage
Room temperature (desiccate)
References
1) Schacter (1999)?A Review of the Antiepileptic Drug Tiagabine, Clin. Neuropharmacol.?22?312 2) Schwartz and Nihalani (2006)?Tiagabine in anxiety disorders, Expert Opin. Pharmacother.?7?1977 3) Novak?et al.?(2001)?Treatment of Painful Sensory Neuropathy With Tiagabine: A Pilot Study, Clin. Auton. Res.?11?357 4) Braestrup?et al.?(1990)?(R)-N-[4,4-bis(3-methyl-2-thienyl)but-3-en-1-yl)nipecotic Acid Binds With High Affinity to the Brain Gamma-Aminobutyric Acid Uptake Carrier, J. Neurochem. 54 639 5) Nielsen?et al.?(1991)?Characterization of Tiagabine (NO-328), a New Potent and Selective GABA Uptake Inhibitor, Eur. J. Pharmacol.?196?257
Properties of Tiagabine hydrochloride
| Melting point: | >192oC dec. |
| alpha | D20 -11° |
| Flash point: | 9℃ |
| storage temp. | 2-8°C |
| solubility | H2O: soluble10mg/mL, clear |
| form | powder |
| pka | pKa1 3.3; pKa2 9.4(at 25℃) |
| color | white to beige |
| optical activity | [α]/D -9 to -12°, c = 1 in H2O |
| Merck | 14,9417 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO, ethanol, or distilled water may be stored at -20° for up to 3 months. |
| CAS DataBase Reference | 145821-59-6(CAS DataBase Reference) |
Safety information for Tiagabine hydrochloride
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H370:Specific target organ toxicity, single exposure |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P311:Call a POISON CENTER or doctor/physician. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Tiagabine hydrochloride
| InChIKey | YUKARLAABCGMCN-PKLMIRHRSA-N |
Tiagabine hydrochloride manufacturer
Apotex Pharmachem India Pvt Ltd
Ralington Pharma
New Products
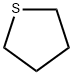
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 145821-59-6 Tiagabine hydrochloride 98%View Details
145821-59-6 Tiagabine hydrochloride 98%View Details
145821-59-6 -
 145821-59-6 98%View Details
145821-59-6 98%View Details
145821-59-6 -
 Tiagabine hydrochloride 98%View Details
Tiagabine hydrochloride 98%View Details
145821-59-6 -
 Tiagabine hydrochloride 145821-59-6 98%View Details
Tiagabine hydrochloride 145821-59-6 98%View Details
145821-59-6 -
 Tiagabine Hydrochloride CAS 145821-59-6View Details
Tiagabine Hydrochloride CAS 145821-59-6View Details
145821-59-6 -
 Tiagabine Hydrochloride CAS 145821-59-6View Details
Tiagabine Hydrochloride CAS 145821-59-6View Details
145821-59-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1