Thioridazine hydrochloride
Synonym(s):10-(2-(1-Methyl-2-piperidyl)ethyl)-2-(methylthio)-10H-phenothiazine, HCl, 10-(2-(1-methylpiperidin-2-yl)ethyl)-2-(methylthio)-10H-phenothiazine, HCl;10-[2-(1-Methyl-2-piperidyl)ethyl]-2-(methylthio)-10H-phenothiazine hydrochloride;Dopamine Receptor Antagonist II, Thioridazine, HCl - CAS 130-61-0 - Calbiochem;Thioridazine hydrochloride
- CAS NO.:130-61-0
- Empirical Formula: C21H27ClN2S2
- Molecular Weight: 407.04
- MDL number: MFCD00012655
- EINECS: 204-992-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
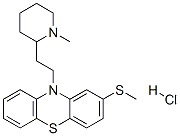
What is Thioridazine hydrochloride?
Chemical properties
Pale Yellow Solid
Originator
Mellaril,Sandoz,US,1959
The Uses of Thioridazine hydrochloride
Thioridazine hydrochloride has been used as an intercalating agent for analyzing the integrity of double-stranded DNA (dsDNA) using square-wave voltammetry (SWV) techniques. Thioridazine hydrochloride has also been used as a positive control for the inhibition of hepatic enzyme cytochrome P4502D6 (CYP2D6) in human liver microsomes.
The Uses of Thioridazine hydrochloride
Dopamine receptor blocker; parent compound of sulforidazine and mesoridazine. Antipsychotic
The Uses of Thioridazine hydrochloride
Thioridazine HCl is a dopamine receptor blocker and antipsychotic. It is the parent compound of sulforidazine and mesoridazine. This compound has been reported to bind strongly to dopamine receptors on cancer stem cells and cause differentiation leaving normal cells alone (see Can. Chem. News 12 July/August 2012).
What are the applications of Application
Thioridazine Hydrochloride is a D2DR, D4DR, and calcium channel protein inhibitor
Definition
ChEBI: Thioridazine hydrochloride is a hydrochloride. It has a role as a first generation antipsychotic and a geroprotector. It contains a thioridazine.
Manufacturing Process
N-(m-methylmercapto-phenyl)-aniline (MP 59° to 61°C) is prepared by
condensing m-methylmercapto-aniline (BP 163° to 165°C/16 mm Hg) with the
potassium salt of o-chloro-benzoic acid and decarboxylating the resultant N-
(m-methylmercapto-phenyl)-anthranilic acid (MP 139° to 141°C) by heating,
and then distilling.
9.87 parts of N-(m-methylmercapto-phenyl)-aniline are heated with 2.93
parts of sulfur and 0.15 part of powdered iodine for 15 minutes in a bath at
about 160°C. Upon termination of the ensuing evolution of hydrogen sulfide,
animal charcoal is added to the reaction mixture and recrystallization carried
out first from 40 parts by volume of chlorobenzene, and then from 25 to 30
parts by volume of benzene at the boiling temperature. The obtained citronyellow
3-methylmercapto-phenothiazine has a MP of 138° to 140°C.
17.82 parts of 2-methylmercapto-phenothiazine, 3.4 parts of finely pulverized sodamide and 80 parts by volume of absolute xylene are heated to boiling for
two hours at a bath temperature of 180°C under a reflux condenser and while
stirring the reaction mixture. Without interrupting the heating, a solution of
13.2 parts of 2-(N-methyl-piperidyl-2')-1chloro-ethane in 40 parts by volume
of absolute xylene is then added dropwise in the course of 1 1/2 hours. After
further heating for 3 hours, the reaction mixture is cooled and, after the
addition of 5 parts of ammonium chloride, is shaken three times with water,
using 25 parts by volume each time. The xylene solution is extracted once
with 35 parts by volume of 3 normal acetic acid and then three times, each
time with 15 parts by volume of the said acid, after which the acetic acid
extract is washed with 60 parts by volume of ether and is then made
phenolphthalein-alkaline by means of 25 parts by volume of concentrated
aqueous caustic soda solution.
The precipitated oily base is taken up in a total of 100 parts by volume of
benzene. The benzene layer, dried over potassium carbonate, is filtered and
then evaporated under reduced pressure. The residue from the evaporation is
distilled in a high vacuum; after separating a preliminary distillate which
passes over up to 228°C under a pressure of 0.92 mm Hg, the principal
fraction, 2-methylmercapto-10-[2'-(N-methyl-piperidyl-2'')-ethyl-
1']phenothiazine, which distills over at 228° to 232°C under the lastmentioned
pressure, is collected. The analytically pure base has a BP of
230°C/0.02 mm Hg.
Therapeutic Function
Tranquilizer
General Description
Thioridazine hydrochloride,10-[2-(1-methyl-2-piperidyl)ethyl]-2-(methylthio)phenothiazine monohydrochloride (Mellaril), is amember of the piperidine subgroup of the phenothiazines.The drug has a relatively low tendency to produce EPS. Thedrug has high anticholinergic activity, and this activity in thestriatum, counterbalancing a striatal DA block, may be responsiblefor the low EPS. It also has been suggested thatthere may be increased DA receptor selectivity, which may be responsible. The drug has sedative and hypotensive activityin common with chlorpromazine and less antiemeticactivity. At high doses, pigmentary retinopathy has been observed.Its major metabolites include N-demethylated, ringhydroxylated,and S-oxidized products. Thioridazine isprominently converted to the active metabolite mesoridazine(discussed next), which probably contributes to the antipsychoticactivity of thioridazine.
Biological Activity
Dopamine receptor antagonist that displays antipsychotic activity.
Biochem/physiol Actions
D2 dopamine receptor antagonist; phenothazine antipsychotic with reduced extrapyramidal side effects; Ca2+ channel blocker.
storage
Desiccate at RT
Properties of Thioridazine hydrochloride
| Melting point: | 158-1600C |
| Boiling point: | 230°C (rough estimate) |
| Density | 1.1227 (rough estimate) |
| refractive index | 1.5610 (estimate) |
| storage temp. | 2-8°C |
| solubility | H2O: soluble250 mg plus 5 ml of solvent, clear, colorless to faintly yellow |
| form | Off-white solid |
| color | White to Almost white |
| Water Solubility | Soluble in water (75 mM), DMSO (100 mM), chloroform, ethanol, and methanol. |
| Sensitive | Light Sensitive & Hygroscopic |
| λmax | 317nm(EtOH)(lit.) |
| Merck | 14,9359 |
| CAS DataBase Reference | 130-61-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Thioridazine hydrochloride(130-61-0) |
| EPA Substance Registry System | 10H-Phenothiazine, 10-[2-(1-methyl-2-piperidinyl)ethyl]-2-(methylthio)-, monohydrochloride (130-61-0) |
Safety information for Thioridazine hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for Thioridazine hydrochloride
| InChIKey | NZFNXWQNBYZDAQ-UHFFFAOYSA-N |
Abamectin manufacturer
Dishman Carbogen Amcis Ltd (Dishman Group)
Meck Pharmaceuticals and Chemicals Pvt. Ltd.
aistrifarma
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran


![2-methylsulfonyl-10-[2-[1-(trideuteriomethyl)piperidin-2-yl]ethyl]phenothiazine](https://img.chemicalbook.in/CAS/20180528/GIF/1329652-09-6.gif)
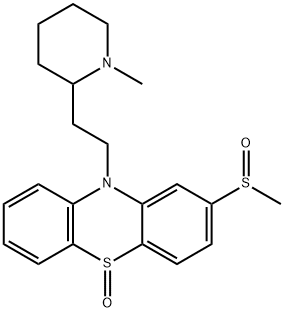
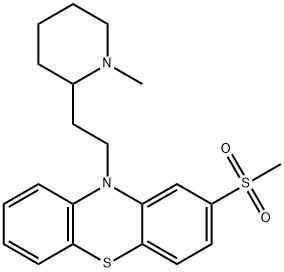
You may like
-
 130-61-0 98%View Details
130-61-0 98%View Details
130-61-0 -
 Thioridazine hydrochloride 98%View Details
Thioridazine hydrochloride 98%View Details
130-61-0 -
 Thioridazine HCl 98% CAS 130-61-0View Details
Thioridazine HCl 98% CAS 130-61-0View Details
130-61-0 -
 Thioridazine hydrochloride 130-61-0 98%View Details
Thioridazine hydrochloride 130-61-0 98%View Details
130-61-0 -
 Thioridazine Hydrochloride CAS 130-61-0View Details
Thioridazine Hydrochloride CAS 130-61-0View Details
130-61-0 -
 Thioridazine hydrochloride CAS 130-61-0View Details
Thioridazine hydrochloride CAS 130-61-0View Details
130-61-0 -
 Thioridazine Hydrochloride CAS 130-61-0View Details
Thioridazine Hydrochloride CAS 130-61-0View Details
130-61-0 -
 THIORIDAZINE HYDROCHLORIDE CAS 130-61-0View Details
THIORIDAZINE HYDROCHLORIDE CAS 130-61-0View Details
130-61-0