Tetramethylthiourea
- CAS NO.:2782-91-4
- Empirical Formula: C5H12N2S
- Molecular Weight: 132.23
- MDL number: MFCD00008324
- EINECS: 220-488-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
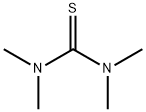
What is Tetramethylthiourea?
Chemical properties
COLORLESS TO YELLOW CRYSTALLINE CHUNKS
The Uses of Tetramethylthiourea
Tetramethylthiourea forms BF3 adduct with tetramethylselenourea.
The Uses of Tetramethylthiourea
As a drug.
Definition
ChEBI: Tetramethylthiourea is a member of thioureas.
General Description
White crystals.
Air & Water Reactions
Water soluble.
Reactivity Profile
An organosulfide amide. Organosulfides are incompatible with acids, diazo and azo compounds, halocarbons, isocyanates, aldehydes, alkali metals, nitrides, hydrides, and other strong reducing agents. Reactions with these materials generate heat and in many cases hydrogen gas. Many of these compounds may liberate hydrogen sulfide upon decomposition or reaction with an acid. Organic amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx).
Hazard
Moderately toxic by ingestion.
Fire Hazard
Flash point data for Tetramethylthiourea are not available, but Tetramethylthiourea is probably combustible.
Safety Profile
Moderately toxic by ingestion. Questionable carcinogen with experimental carcinogenic and teratogenic data. Human mutation data reported. When heated to decomposition it emits very toxic fumes of NOx and SOx. See also SULFIDES.
Properties of Tetramethylthiourea
| Melting point: | 75-77 °C (lit.) |
| Boiling point: | 245 °C (lit.) |
| Density | 1.005 (estimate) |
| refractive index | 1.5300 (estimate) |
| Flash point: | 190 °C |
| storage temp. | 2-8°C |
| solubility | 32g/l |
| pka | 1.50±0.50(Predicted) |
| form | solid |
| color | Off-White to Pale Beige |
| Water Solubility | 32 g/L (20 ºC) |
| BRN | 1744916 |
| CAS DataBase Reference | 2782-91-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Thiourea, tetramethyl-(2782-91-4) |
| EPA Substance Registry System | Tetramethylthiourea (2782-91-4) |
Safety information for Tetramethylthiourea
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for Tetramethylthiourea
| InChIKey | MNOILHPDHOHILI-UHFFFAOYSA-N |
Related products of tetrahydrofuran

You may like
-
 N,N,N',N'-Tetramethylthiourea CAS 2782-91-4View Details
N,N,N',N'-Tetramethylthiourea CAS 2782-91-4View Details
2782-91-4 -
 Tetramethylthiourea 98% CAS 2782-91-4View Details
Tetramethylthiourea 98% CAS 2782-91-4View Details
2782-91-4 -
 Tetramethylthiourea, 98% CAS 2782-91-4View Details
Tetramethylthiourea, 98% CAS 2782-91-4View Details
2782-91-4 -
 Tetramethylthiourea CAS 2782-91-4View Details
Tetramethylthiourea CAS 2782-91-4View Details
2782-91-4 -
 Tetramethylthiourea CAS 2782-91-4View Details
Tetramethylthiourea CAS 2782-91-4View Details
2782-91-4 -
 106-43-4 Para Chloro Toluene (PCT) 99.00%View Details
106-43-4 Para Chloro Toluene (PCT) 99.00%View Details
106-43-4 -
 Pyrrolidine 99.00%View Details
Pyrrolidine 99.00%View Details
123-75-1 -
 12029-98-0 99.00%View Details
12029-98-0 99.00%View Details
12029-98-0