Tetramethylsilane
Synonym(s):Tetramethylsilane;TMS
- CAS NO.:75-76-3
- Empirical Formula: C4H12Si
- Molecular Weight: 88.22
- MDL number: MFCD00008274
- EINECS: 200-899-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
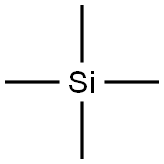
What is Tetramethylsilane?
Description
Tetramethylsilane (TMS1) is the simplest tetraalkylsilane and the organosilicon analogue of neopentane. It is a volatile liquid with a characteristic “organometallic” odor.
TMS has been known since at least 1911, when Artur Bygden at the University of Uppsala (Sweden) prepared it from silicon tetrachloride and the Grignard reagent2 methylmagnesium chloride. Nowadays, TMS is made less expensively, as one of the products of the direct alkylation of elemental silicon with chloromethane.
TMS is sometimes used as a starting material for synthesizing more complex organosilanes, but its main use by far is as a versatile internal standard in NMR spectroscopy. Its 1H, 13C, and 29Si signals are sharp and have resonances that are distinctly different from the nuclei normally encountered in test samples.
For example, the 1H atoms in TMS are highly shielded by the silicon atom and therefore resonate at a higher magnetic field than 1H atoms surrounded by more common nuclei such as carbon, oxygen, and nitrogen. TMS is such a common standard that its 1H chemical shift (δ) is defined as zero. In most 1H spectra, all of the signals are to the left (lower field) of the TMS signal, although highly shielded protons in some aromatic compounds can appear to the right of TMS.
Highly soluble TMS is an ideal standard for generating NMR spectra of samples in organic solvents, but its low water solubility makes it unusable for aqueous samples. TMS’ low cost, chemical inertness, and ease of removal because of its volatility are additional factors that make it the universal standard?for organic-solution NMR.
1. TMS is also used as the abbreviation for the trimethylsilyl radical.
2. Victor Grignard, the French Nobel Prize–winning chemist, had recently discovered the eponymous reaction; and chemists around the world were experimenting with alkylmagnesium halides in all sorts of ways.
Chemical properties
Colorless clear liquid
The Uses of Tetramethylsilane
NMR reference standard. In semiconductor applications (chemical vapor deposition). Tetramethylsilane (TMS) is used as a chemical shift reference for proton, carbon-13, and silicon-29 analysis in organic solvents and is given 0 as its chemical shift position.
The Uses of Tetramethylsilane
Tetramethylsilane is used as a building block in organometallic chemistry. It acts as a by-product in the production of methyl chlorosilanes. Also, it serves as a precursor to silicon dioxide or silicon carbide. It is used as internal reference standard for the calibration of chemical sift for 1, 13 and 29 NMR spectroscopy. In addition, it is used as an aviation fuel.
Definition
ChEBI: Tetramethylsilane is an organosilicon compound that is silane in which the hydrogens have been replaced by methyl groups.
General Description
Tetramethylsilane appears as a colorless, mildly acidic volatile liquid. A serious fire hazard. Mildly toxic by ingestion. Emits acrid smoke and fumes when heated to high temperatures. Less dense than water and insoluble in water, but soluble in most organic solvents. Used as an aviation fuel and as an internal standard for nmr analytical instruments.
Air & Water Reactions
Highly flammable. Tetramethylsilane is insoluble in water.
Reactivity Profile
Hydrides, such as Tetramethylsilane, are reducing agents and react rapidly and dangerously with oxygen and with other oxidizing agents, even weak ones. Thus, they are likely to ignite on contact with alcohols. Hydrides are incompatible with acids, alcohols, amines, and aldehydes.
Hazard
Flammable, high fire risk.
Health Hazard
May cause toxic effects if inhaled or absorbed through skin. Inhalation or contact with material may irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Purification Methods
Distil it from conc H2SO4 (after shaking with it) or LiAlH4, through a 5ft vacuum-jacketed column packed with glass helices into an ice-cooled condenser, then percolate it through silica gel to remove traces of halide. [Beilstein 4 IV 3875.]
Properties of Tetramethylsilane
| Melting point: | -99 °C (lit.) |
| Boiling point: | 26-28 °C (lit.) |
| Density | 0.648 g/mL at 25 °C (lit.) |
| vapor pressure | 11.66 psi ( 20 °C) |
| refractive index | n |
| RTECS | VV5706000 |
| Flash point: | −17 °F |
| storage temp. | 2-8°C |
| solubility | 0.02g/l |
| form | liquid |
| appearance | colorless liquid |
| color | colorless |
| Specific Gravity | 0.648 |
| explosive limit | 1.0-37.9%(V) |
| Water Solubility | 0.02 G/L (25 ºC) |
| Hydrolytic Sensitivity | 1: no significant reaction with aqueous systems |
| Merck | 14,9229 |
| BRN | 1696908 |
| Dielectric constant | 1.9199999999999999 |
| Stability: | Stable. Highly flammable - readily forms explosive mixtures with air. Note the boiling point close to room temperature, the low flash point and high vapour pressure. Incompatible with oxidizing agents. Refrigerate before opening. |
| CAS DataBase Reference | 75-76-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Silane, tetramethyl-(75-76-3) |
| EPA Substance Registry System | Tetramethylsilane (75-76-3) |
Safety information for Tetramethylsilane
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H224:Flammable liquids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P403+P233:Store in a well-ventilated place. Keep container tightly closed. |
Computed Descriptors for Tetramethylsilane
| InChIKey | CZDYPVPMEAXLPK-UHFFFAOYSA-N |
Related products of tetrahydrofuran


You may like
-
 75-76-3 Tetramethyl silane 99%View Details
75-76-3 Tetramethyl silane 99%View Details
75-76-3 -
![Tetramethylsilane [for NMR] CAS 75-76-3](https://img.chemicalbook.in//Content/image/CP5.jpg) Tetramethylsilane [for NMR] CAS 75-76-3View Details
Tetramethylsilane [for NMR] CAS 75-76-3View Details
75-76-3 -
 Tetramethylsilane CAS 75-76-3View Details
Tetramethylsilane CAS 75-76-3View Details
75-76-3 -
 T.M.S (TETRAMETHYL SILANE) in glass bottle 99% CAS 75-76-3View Details
T.M.S (TETRAMETHYL SILANE) in glass bottle 99% CAS 75-76-3View Details
75-76-3 -
 106-43-4 Para Chloro Toluene (PCT) 99.00%View Details
106-43-4 Para Chloro Toluene (PCT) 99.00%View Details
106-43-4 -
 Pyrrolidine 99.00%View Details
Pyrrolidine 99.00%View Details
123-75-1 -
 Orthochlorobenzaldehyde (2-Chlorobenzaldehyde) 89-98-5 99.00%View Details
Orthochlorobenzaldehyde (2-Chlorobenzaldehyde) 89-98-5 99.00%View Details
89-98-5 -
 12029-98-0 99.00%View Details
12029-98-0 99.00%View Details
12029-98-0