Tetramethylammonium fluoride
Synonym(s):TMAF
- CAS NO.:373-68-2
- Empirical Formula: C4H12FN
- Molecular Weight: 93.14
- MDL number: MFCD00011627
- EINECS: 206-769-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-16 09:55:26
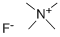
What is Tetramethylammonium fluoride?
Description
Tetramethylammonium fluoride is the quaternary ammonium salt with the formula (CH3)4NF. This hygroscopic white solid is a source of "naked fluoride", that is fluoride ions not connected to a metal atom in a complex. Most other soluble salts of fluoride are in fact bifluorides, HF2–. Historically, there has been two main approaches to prepare TMAF:
(i) Via neutralization of tetramethylammonium hydroxide (TMAOH) with HF.
(ii) through the metathesis reaction of different ammonium salts with inorganic sources of fluoride such as KF or CsF.
Due to the high basicity of the fluoride anion, the salt reacts slowly with acetonitrile, inducing its dimerization to CH3C(NH2)=CHCN, which co-crystallizes.
The Uses of Tetramethylammonium fluoride
Tetramethylammonium fluoride may be used in combination with sulfuryl fluoride for the conversion of aryl phenols to aryl fluorides. It can react with aminosilanes to generate onium amide bases in situ, which can deprotonate heteroarenes.
The Uses of Tetramethylammonium fluoride
Reactant for:
- Halide-induced ring opening of 2-substituted aziridinium salts
- Synthesis of fluoro aromatics via fluorodenitration reactions
- Synthesis of sevoflurane in ionic liquids by halogen-exchange fluorination
- Synthesis of fluorinated Poly(oxomolybdates)
What are the applications of Application
Tetramethylammonium fluoride is useful for halogen exchange
Properties of Tetramethylammonium fluoride
| Melting point: | 170 °C (dec.) (lit.) |
| CAS DataBase Reference | 373-68-2(CAS DataBase Reference) |
| EPA Substance Registry System | Tetramethylammonium fluoride (373-68-2) |
Safety information for Tetramethylammonium fluoride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Tetramethylammonium fluoride
| InChIKey | HQFTZNVQVRRDLN-UHFFFAOYSA-M |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


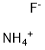
You may like
-
 Tetramethylammonium fluoride CASView Details
Tetramethylammonium fluoride CASView Details -
 Tetramethylammonium fluoride CAS 373-68-2View Details
Tetramethylammonium fluoride CAS 373-68-2View Details
373-68-2 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
