TETRAIODOPHTHALIC ANHYDRIDE
- CAS NO.:632-80-4
- Empirical Formula: C8I4O3
- Molecular Weight: 651.7
- MDL number: MFCD00083098
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-16 09:55:26
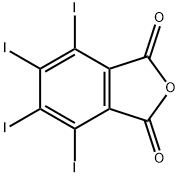
What is TETRAIODOPHTHALIC ANHYDRIDE?
Preparation
CAUTION: This reaction should be carried out in a well-ventilated hood. <br/>To a flask equipped with a mechanical stirrer and an air condenser topped with a tube leading to a gas trap is added 74.0 gm (0.5 mole) of phthalic anhydride, 162 gm (0.638 mole) of iodine, and 300 ml of 60% fuming sulfuric acid (1.84 moles). The flask is gently heated to 45-50°C, at which point the reaction commences. If the reaction becomes too vigorous it may be necessary to use a ice bath to lower the temperature to 40-50°C. The reaction mixture is eventually (4 hr) heated up to 65°C until all visible reaction has ceased. The reaction mixture is cooled to 10-20°C and an additional 81.0 gm (0.318 mole) of iodine is added and the reaction again slowly heated up to 65°C (1?hr) and again when the reaction ceases it is cooled. Another 27.0 gm (0.107 mole) of iodine is added and the reaction is again heated up to 65°C (1 hr). The flask is heated with an oil bath to a bath temperature of 175-180°C, at which point the sulfur trioxide and iodine fumes evolve. After about 2 hr, when the gaseous evolution ceases, the flask is cooled to about 60°C and the mxiture then poured into a beaker of water. The contents are allowed to stand overnight at room temperature, filtered, washed with two 50-ml portions of cone, sulfuric acid and then with three 100 ml portions of water. The light yellow crystalline product is put into a beaker containing 1 liter of water and 10 gm of sodium bisulfite in order to remove the last traces of free iodine. The aqueous solution is decanted, the product washed five times with ? liter of water, washed twice with 100 ml of acetone and then dried at 60°C to afford 260-268 gm (80-82%), m.p. 327-328°C.
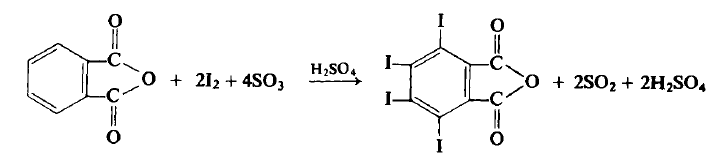
Properties of TETRAIODOPHTHALIC ANHYDRIDE
| Melting point: | 327.5°C |
| Boiling point: | 584.2±50.0 °C(Predicted) |
| Density | 3.1538 (estimate) |
| EPA Substance Registry System | 1,3-Isobenzofurandione, 4,5,6,7-tetraiodo- (632-80-4) |
Safety information for TETRAIODOPHTHALIC ANHYDRIDE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for TETRAIODOPHTHALIC ANHYDRIDE
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Potassium Hydroxide 90%View Details
Potassium Hydroxide 90%View Details
1310-58-3 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
