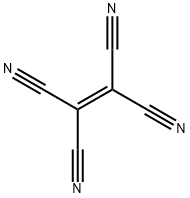
Tetracyanoethylene
Synonym(s):Ethylenetetracarbonitrile;NSC 24833;Percyanoethylene;TCNE
- CAS NO.:670-54-2
- Empirical Formula: C6N4
- Molecular Weight: 128.09
- MDL number: MFCD00001850
- EINECS: 211-578-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
What is Tetracyanoethylene?
Description
Tetracyanoethylene (TCNE) is a crystalline solid with a melting point of 200 °C. DuPont researchers prepared it?in 1957 by treating dibromomalononitrile?with copper in boiling benzene. TCNE is an excellent electron acceptor and has been used to prepare organic superconductors.
In 2001, J. S. Miller and co-workers at the University of Utah (Salt Lake City) discovered that the TCNE radical anion forms a dianionic dimer. They believed that the dimer contains an unusual four-atom, two-electron bond that connects the four olefinic carbon atoms. Recently, Miller and colleagues used Raman spectroscopy to confirm?the existence of the four-center bond.
Chemical properties
white to beige-brown crystalline powder, crystals. Tetracyanoethylene [670-54-2], ethenetetracarbonitrile, TCNE, ( NC)2C = C (CN)2, Mr 128.09, mp 197 – 198℃, bp 223℃, n25 D 1.560. The preferred synthetic preparation of TCNE involves the debromination of the KBr complex of dibromomalononitrile. Tetracyanoethylene is a reactive compound that undergoes a variety of reactions including addition, replacement and cyclization. The chemistry of TCNE is exhaustively reviewed in.
The Uses of Tetracyanoethylene
In the synthesis of spiro Compounds, in modified Diels-Alder reactions, as aromatizing agent: Longone, Smith, Tetrahedron Lett. 1962, 205.
The Uses of Tetracyanoethylene
Used for postfunctional addition to polyphenylacetylene derivatives to change the oxygen permeability. As reactant for Regioselective [2+2] cycloaddition reaction for production of BODIPY dyes2 and TCBD derivatives, Thermal addition reaction with alkynes, One-pot reactions with nucleophilic reagents forming aromatic cyanovinyl compounds, Synthesis of cobalt tetracyanoethylene films, Biotransformation by Botrytis cinerea.
The Uses of Tetracyanoethylene
Used for postfunctional addition to polyphenylacetylene derivatives to change the oxygen permeability
Reactant for:
- Regioselective [2+2] cycloaddition reaction for production of BODIPY dyes and TCBD derivatives
- Thermal addition reaction with alkynes
- One-pot reactions with nucleophilic reagents forming aromatic cyanovinyl compounds
- Synthesis of cobalt tetracyanoethylene films
- Biotransformation by Botrytis cinerea
What are the applications of Application
Tetracyanoethylene is Used for postfunctional addition to polyphenylacetylene derivatives to change the oxygen permeability
Definition
The first member of a class of compounds called cyanocarbons.
Synthesis Reference(s)
Organic Syntheses, Coll. Vol. 4, p. 877, 1963
The Journal of Organic Chemistry, 45, p. 5113, 1980 DOI: 10.1021/jo01313a019
Hazard
Hydrolyzes in moist air to hydrogen cyanide.
Purification Methods
Crystallise it from chlorobenzene, dichloroethane, or dichloromethane [Hall et al. J Org Chem 52 5528 1987]. Storeitat0oina desiccator over NaOH pellets. (It slowly evolves HCN on exposure to moist air CARE.) It can also be sublimed at 120o under vacuum. Also purify it by repeated sublimation at 120-130o/0.5mm. [Frey et al. J Am Chem Soc 107 748 1985, Traylor & Miksztal J Am Chem Soc 109 2778 1987, Fatiadi Synthesis 249 1986, Synthesis 749 1967, Beilstein 2 IV 1245.]
Properties of Tetracyanoethylene
| Melting point: | 197-199 °C(lit.) |
| Boiling point: | 223 °C |
| Density | 1,348 g/cm3 |
| refractive index | 1.5600 |
| Flash point: | 223°C |
| storage temp. | 2-8°C |
| form | Crystalline Powder, Crystals or Chunks |
| color | White to beige-brown |
| Water Solubility | hydrolyzes |
| Sensitive | Moisture Sensitive |
| λmax | 328nm(CHCl3)(lit.) |
| Merck | 14,9195 |
| BRN | 1679885 |
| CAS DataBase Reference | 670-54-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Tetracyanoethylene(670-54-2) |
| EPA Substance Registry System | Ethenetetracarbonitrile (670-54-2) |
Safety information for Tetracyanoethylene
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H300:Acute toxicity,oral |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Tetracyanoethylene
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
![3,4-Dithia-7H-cyclopenta[a]pentalene](https://img.chemicalbook.in/CAS/GIF/389-58-2.gif)
You may like
-
 Tetracyanoethylene CAS 670-54-2View Details
Tetracyanoethylene CAS 670-54-2View Details
670-54-2 -
 Tetracyanoethylene CAS 670-54-2View Details
Tetracyanoethylene CAS 670-54-2View Details
670-54-2 -
 Tetracyanoethylene (purified by sublimation) CAS 670-54-2View Details
Tetracyanoethylene (purified by sublimation) CAS 670-54-2View Details
670-54-2 -
 Tetracyanoethylene CAS 670-54-2View Details
Tetracyanoethylene CAS 670-54-2View Details
670-54-2 -
 Tetracyanoethylene 98% (GC) CAS 670-54-2View Details
Tetracyanoethylene 98% (GC) CAS 670-54-2View Details
670-54-2 -
 Tetracyanoethylene CAS 670-54-2View Details
Tetracyanoethylene CAS 670-54-2View Details
670-54-2 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
