Tetracaine
Synonym(s):4-(Butylamino)benzoic acid 2-(dimethylamino)ethyl ester;Tetracaine
- CAS NO.:94-24-6
- Empirical Formula: C15H24N2O2
- Molecular Weight: 264.36
- MDL number: MFCD00053787
- EINECS: 202-316-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
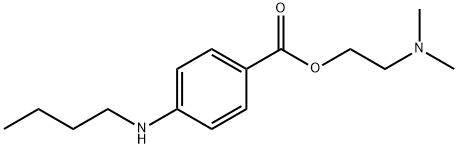
What is Tetracaine?
Absorption
The systemic absorption of anesthetic from a combination cream is influenced by two main factors: the duration of application and the surface area it covers on the skin. In a study where peak plasma concentrations were measured for the anesthetic agents within the cream, it was found that while the levels of lidocaine could be successfully determined, the plasma levels for tetracaine remained undetectable due to their extremely low concentration, which was less than 0.9 ng/mL. This suggests that the presence of tetracaine in the bloodstream, if any, was below the threshold required for accurate measurement.
Toxicity
The most common adverse effects with the combination cream are localized reactions such as: erythema (47%), skin discoloration (16%), and edema (14%). Systemic adverse events were less common, occurring at a rate of <1% and included vomiting, headache, dizziness, and fever. Similar to other amide and ester anesthetics, CNS excitation and/or depression may occur. It is not well known at which plasma concentration systemic toxicity occurs with tetracaine; however, the threshold is thought to be much lower than that of lidocaine which is 1000 ng/mL.
Chemical properties
White or light-yellow, waxy solid. Very slightly soluble in water; soluble in alcohol, ether, benzene, chloroform.
The Uses of Tetracaine
Tetracaine, also known as amethocaine, is the most potent and lipidsoluble ester local anaesthetic and has significant potential for toxicity if used systemically. It is commonly used as a 4% gel for topical application to decrease the pain from venous puncture or cannulation.
The Uses of Tetracaine
analgesic
The Uses of Tetracaine
Tetracaine has been used as an anesthetic and to lower the glucose synthesis in upper small intestinal infusions. It has also been used to inhibit ryanodine receptors (RyRs) in rats.
The Uses of Tetracaine
Topical ophthalmic anesthetic; used for spinal anesthesia
What are the applications of Application
Tetracaine is an allosteric blocker of Ca2+ release at ryanodine receptors
Indications
Tetracaine, an ester local anesthetic, is commonly combined with lidocaine to form a cream and patch for various medical applications.
Specifically, the ophthalmic formulation of tetracaine is used for procedures that necessitate a rapid and short-acting topical anesthetic effect in the eye. Its quick onset and brief duration make it suitable for sensitive ocular applications.
The lidocaine and tetracaine combination patch is designed for local dermal analgesia, particularly for superficial dermatological procedures and for accessing superficial veins. This patch provides a targeted and effective means of numbing the skin before minor skin procedures or venipuncture.
Similarly, the lidocaine and tetracaine cream is intended to offer topical local analgesia for a range of superficial dermatological procedures. The cream formulation allows for easy application and coverage of the area requiring anesthesia, ensuring patient comfort during minor skin interventions.
Definition
ChEBI: A benzoate ester in which 4-N-butylbenzoic acid and 2-(dimethylamino)ethanol have combined to form the ester bond; a local ester anaesthetic (ester caine) used for surface and spinal anaesthesia.
Biological Functions
Tetracaine hydrochloride (Pontocaine) is an ester of PABA that is an effective topical local anesthetic agent and also is quite commonly used for spinal (subarachnoid) anesthesia. Epinephrine is frequently added to prolong the anesthesia.Tetracaine is considerably more potent and more toxic than procaine and cocaine. It has approximately a 5-minute onset and 2 to 3 hours of action.
General Description
Tetracaine was developed to address the low potency andshort duration of action of procaine and chloroprocaine. The addition of the butyl side chain on the para nitrogen increasesthe lipid solubility of the drug and enhances the topical potencyof tetracaine. The plasma half-life is 120 to 150 seconds.Topically applied tetracaine to unbroken skin requires 30 to 45minutes to confer topical anesthesia. Tetracaine 4% gel is superiorthan eutectic mixture with lidocaine (EMLA) (an emulsionof lidocaine and prilocaine) in preventing pain associatedwith needle procedures in children. Tetracaine metabolism issimilar to procaine ester metabolism yielding parabutylaminobenzoicacid and dimethylaminoethanol and conjugatesexcreted in the urine. The pKa of the dimethylated nitrogen is8.4 and tetracaine is formulated as a hydrochloride salt with apH of 3.5 to 6.0. The increased absorption from topical siteshas resulted in reported toxicity. Overdoses of tetracaine mayproduce central nervous system (CNS) toxicity and seizure activitywith fatalities from circulatory depression reported.No selective cardiac toxicity is seen with tetracaine althoughhypotension has been reported. Tetracaine is employed for infiltrationanesthesia, spinal anesthesia, or topical use.
Biochem/physiol Actions
Tetracaine also refers as 4-(Butylamino)benzoic acid 2-(dimethylamino)ethyl ester interfere with calcium movement in muscle and non-muscle cells and can inhibit potassium-induced as well as caffeine-induced shortening of outer hair cells (OHC′s). But, the product can′t inhibit electrically-induced shortening of OHC′s.The product can also stimulate caspase activation and inhibits pro-survival signalling pathways which in turn induce human renal cell apoptosis.
Veterinary Drugs and Treatments
Tetracaine is more irritating than proparacaine but is sometimes used in veterinary medicine. It is indicated to produce local anesthesia of short duration for ophthalmic procedures including measurement of intraocular pressure (tonometry), removal of foreign bodies and sutures, and conjunctival and corneal scraping in diagnosis and gonioscopy. Tetracaine is also indicated to produce local anesthesia prior to surgical procedures in humans such as cataract extraction and pterygium excision, usually as an adjunct to locally injected anesthetics. Ophthalmic solutions used for intraocular procedures should be preservative-free. Preservatives may cause damage to the corneal epithelium if a significant quantity of solution enters the eye through the incision.
Metabolism
Tetracaine is rapidly hydrolyzed by plasma esterases to the following primary metabolites: para-aminobenzoic acid and diethylaminoethanol. The activity of both metabolites is unspecified.
Properties of Tetracaine
| Melting point: | 41.0 to 45.0 °C |
| Boiling point: | 407.59°C (rough estimate) |
| Density | 1.0200 (rough estimate) |
| refractive index | 1.5800 (estimate) |
| storage temp. | 2-8°C |
| solubility | soluble in Methanol |
| form | powder to crystal |
| pka | pKa 8.33±0.03(H2O
t = 20.0
I = 0.10 (KCl)) (Uncertain) |
| color | White to Almost white |
| Water Solubility | 156mg/L(temperature not stated) |
| InChI | InChI=1S/C15H24N2O2/c1-4-5-10-16-14-8-6-13(7-9-14)15(18)19-12-11-17(2)3/h6-9,16H,4-5,10-12H2,1-3H3 |
| CAS DataBase Reference | 94-24-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Tetracaine(94-24-6) |
Safety information for Tetracaine
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H317:Sensitisation, Skin H351:Carcinogenicity |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Tetracaine
| InChIKey | GKCBAIGFKIBETG-UHFFFAOYSA-N |
| SMILES | C(OCCN(C)C)(=O)C1=CC=C(NCCCC)C=C1 |
Tetracaine manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
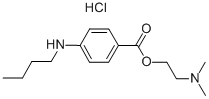

You may like
-
 Amethocaine 98%View Details
Amethocaine 98%View Details -
 Amethocaine 98%View Details
Amethocaine 98%View Details
94-24-6 -
 94-24-6 98%View Details
94-24-6 98%View Details
94-24-6 -
 Tetracaine 98% (GC) CAS 94-24-6View Details
Tetracaine 98% (GC) CAS 94-24-6View Details
94-24-6 -
 Tetracaine CAS 94-24-6View Details
Tetracaine CAS 94-24-6View Details
94-24-6 -
 Tetracaine CAS 94-24-6View Details
Tetracaine CAS 94-24-6View Details
94-24-6 -
 Tetracaine CAS 94-24-6View Details
Tetracaine CAS 94-24-6View Details
94-24-6 -
 TetracaineView Details
TetracaineView Details
94-24-6
