Tetrabutylammonium iodide
Synonym(s):TBAI;Tetra-n-butylammonium iodide
- CAS NO.:311-28-4
- Empirical Formula: C16H36IN
- Molecular Weight: 369.37
- MDL number: MFCD00011636
- EINECS: 206-220-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-10 14:00:07
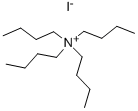
What is Tetrabutylammonium iodide?
Chemical properties
Tetrabutylammonium iodide is an organic ammonium compound with the molecular formula C16H36IN. white or tan powder. Soluble in water and ethanol, slightly soluble in chloroform and benzene. Stable under normal temperature and pressure.
The Uses of Tetrabutylammonium iodide
Tetrabutylammonium iodide is used in the preparation of novel quaternary amines to serve as antibacterial agents in the rise of drug-resistant bacteria,it is also used in phosphonium reversible inhibitors of cholinesterases.
The Uses of Tetrabutylammonium iodide

To a solution of the indole (A) (6.0 g, 22.9 mmol) in dry DMF (60 mL) was added K2CO3 (9.52 g, 68.9 mmol) and the mixture was stirred under N2 at RT for 30 min. To this mixture was added the alkyl bromide (B) (5.5 g, 45.9 mmol) and the reaction was stirred at 80 C for 4 h. After completion, the reaction mixture was cooled to RT and diluted with EtOAc (50 mL). The mixture was washed with brine (50 mL), then cold H2O (50 mL). The org layer was dried (Na2SO4) and concentrated in vacuo. The resulting material was purified by silica gel column chromatography to provide the product as an off-white solid. [6.0 g, 87%]
What are the applications of Application
Tetrabutylammonium iodide may be used as a mobile phase additive in ion-pair high-performance liquid chromatography (IP-HPLC) assay of 4-aminopyridine in serum. It may also be used as a mobile phase additive in the analysis of tetracycline by reversed-phase IPC. The addition of tetrabutylammonium iodide regulates the retention of tetracyclines.
Tetrabutylammonium iodide can be used:
As an additive in the synthesis of fused triazole derivatives using palladium catalyst.
To prepare allyl-PEG-allyl, which is a key intermediate polymer used to synthesize fluorinated amphiphilic copolymers.
As a catalyst used in the synthesis of ethers.
Reactions
Tetrabutylammonium iodide (TBAI) has been used as a catalyst in the following reactions:
Synthesis of O-benzyl-N-Boc-L-tyrosine benzyl ester from N-Boc-L-tyrosine.
Conversion of 8-fluoro-1-aminonaphthalene into 1-(8-fluoro-naphthalen-1-yl)piperazine hydrochloride.
Synthesis of 1-(2,4-dichlorophenyl)-5-(4-(4-iodobut-1-ynyl)phenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide from 4-(4-(1-(2,4-dichlorophenyl)-4-methyl-3-(piperidin-1-ylcarbamoyl)-1H-pyrazol-5-yl)phenyl)but-3-yn-1-yl methanesulfonate.
Other reactions where TBAI can be used as a catalyst:
TBAI-tert-butyl hydroperoxide system can catalyze the conversion of α-methyl styrene derivatives into allylic sulfones by reacting with sulfonylhydrazides under metal-free conditions.
Palladium(0)-catalyzed cross-coupling between benzylic zinc bromides and aryl or alkenyl triflates.
Three-component coupling of amines, carbon dioxide, and halides to form carbamates in the presence of cesium carbonate.
General Description
Tetrabutylammonium iodide is a quaternary ammonium salt used in phase-transfer reactions. It is also used in regioselective ether cleavage reactions and as a source of iodide for nucleophilic displacement reactions.
Flammability and Explosibility
Not classified
Purification Methods
Crystallise the iodide from toluene/pet ether (see entry for the corresponding bromide), acetone, ethyl acetate, EtOH/diethyl ether, nitromethane, aqueous EtOH or water. Dry it at room temperature under a vacuum. It has also been dissolved in MeOH/acetone (1:3, 10mL/g), filtered and allowed to stand at room temperature to evaporate to ca half its original volume. Distilled water (1mL/g) is then added, and the precipitate is filtered off and dried. It can also be dissolved in acetone, precipitated by adding ether and dried in a vacuum at 90o for 2 days. It has also been recrystallised from CH2Cl2/pet ether or hexane, or anhydrous methanol and stored in a vacuum desiccator over H2SO4. [Chau & Espenson J Am Chem Soc 108 1962 1986, Beilstein 4 IV 558.]
Properties of Tetrabutylammonium iodide
| Melting point: | 141-143 °C(lit.) |
| Boiling point: | 145.3℃[at 101 325 Pa] |
| Density | 1.20 |
| vapor pressure | 0Pa at 25℃ |
| storage temp. | Store below +30°C. |
| solubility | acetonitrile: 0.1 g/mL, clear, colorless |
| form | Crystalline Powder |
| color | White to cream |
| Odor | Amine like |
| Water Solubility | Soluble in water and methanol. Insoluble in benzene. |
| Sensitive | Light Sensitive & Hygroscopic |
| λmax | λ: 290 nm Amax: 0.1 λ: 300 nm Amax: 0.05 λ: 320 nm Amax: 0.02 λ: 500 nm Amax: 0.02 |
| BRN | 3916152 |
| Exposure limits | ACGIH: TWA 0.01 ppm |
| Stability: | Stable. Incompatible with strong oxidizing agents. Light-sensitive. |
| CAS DataBase Reference | 311-28-4(CAS DataBase Reference) |
| EPA Substance Registry System | Tetrabutylammonium iodide (311-28-4) |
Safety information for Tetrabutylammonium iodide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P501:Dispose of contents/container to..… |
Computed Descriptors for Tetrabutylammonium iodide
| InChIKey | DPKBAXPHAYBPRL-UHFFFAOYSA-M |
Tetrabutylammonium iodide manufacturer
JSK Chemicals
Pat Impex
Akshaya Pure Chemicals
ASM Organics
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 Tetrabutylammonium iodide 99%View Details
Tetrabutylammonium iodide 99%View Details -
 Tetrabutylammonium Iodide CAS 311-28-4View Details
Tetrabutylammonium Iodide CAS 311-28-4View Details
311-28-4 -
 Tetra-N-Butylammonium Iodide CAS 311-28-4View Details
Tetra-N-Butylammonium Iodide CAS 311-28-4View Details
311-28-4 -
 Tetrabutylammonium Iodide (TBAI) extrapure AR CAS 311-28-4View Details
Tetrabutylammonium Iodide (TBAI) extrapure AR CAS 311-28-4View Details
311-28-4 -
 Tetrabutylammonium IodideView Details
Tetrabutylammonium IodideView Details
311-28-4 -
 Tetra Butyl Ammonium IodideView Details
Tetra Butyl Ammonium IodideView Details
311-28-4 -
 TETRA BUTYL AMMONIUM IODIDE (TBAI), Purity: 99View Details
TETRA BUTYL AMMONIUM IODIDE (TBAI), Purity: 99View Details
311-28-4 -
 Tetrabutylammonium Iodide, Grade Standard: Analytical Grade, Packaging Type: DrumView Details
Tetrabutylammonium Iodide, Grade Standard: Analytical Grade, Packaging Type: DrumView Details
311-28-4
