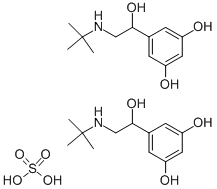
TERBUTALINE SULFATE
Synonym(s):2-t-Butylamino-1-(3,5-dihydroxyphenyl)ethanol;5-[2-[(1,1-Dimethylethyl)amino]-1-hydroxyethyl]-1,3-benzenediol hemisulfate salt;Terbutaline hemisulfate salt
- CAS NO.:23031-32-5
- Empirical Formula: C24H40N2O10S
- Molecular Weight: 548.65
- MDL number: MFCD00079584
- EINECS: 245-386-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-20 16:56:14
What is TERBUTALINE SULFATE?
Description
Terbutaline is the N-t-butyl analogue of metaproterenol and, as such, would be expected to have a more potent β2-selectivity. When compared to metaproterenol, terbutaline has a threefold greater potency at the β2-receptor. Like metaproterenol, it is resistant to COMT and slowly metabolized by MAO, therefore having good oral bioavailability with similar onset and duration. Terbutaline is available as tablets and solutions for injection and inhalation. Adverse effects are similar to other direct-acting β2-selective agonists, however, with a greater incidence of palp
Description
Terbutaline (hemisulfate) (Item No. 21292) is an analytical reference standard categorized as a β2-adrenergic receptor agonist. Terbutaline has been used to enhance physical performance in athletes. This product is intended for use in analytical forensic applications. This product is also available as a general research tool .
Chemical properties
White or almost white, crystalline powder.
Originator
Bricanyl,Pharma-Stern,W. Germany,1971
The Uses of TERBUTALINE SULFATE
Terbutaline Sulfate is derived from the starting material, Terbutaline Hemisulfate Salt (T109750), which is a β-adrenergic receptor agonist. A Bronchodilator; Metabolite of Terbutaline (T109763).
The Uses of TERBUTALINE SULFATE
A B-Adrenergic receptor agonist. A Bronchodilator
The Uses of TERBUTALINE SULFATE
betaadrenergic agonist, bronchodilator
The Uses of TERBUTALINE SULFATE
Anti Asthmatic
What are the applications of Application
Terbutaline Hemisulfate Salt is a β-adrenergic receptor agonist
What are the applications of Application
Terbutaline Hemisulfate is an agonist at β2-adrenergic receptors
Definition
ChEBI: Terbutaline sulfate is an ethanolamine sulfate salt. It is functionally related to a terbutaline.
Manufacturing Process
To a solution of 32 g of benzyl-t-butylamine in 300 ml of absolute ethanol at
reflux temperature was added 32 g of 3,5-dibenzyloxy-?-bromoacetophenone
in 10 ml of dry benzene. The mixture was refluxed for 20 hours and then
evaporated. When absolute ether was added to the residue, benzyl-tbutylamine
hydrobromide was precipitated. The precipitated compound was
filtered off and to the filtrate was added an excess of 2 N sulfuric acid. This
caused precipitation of the hydrogen sulfate of 3,5-dibenzyloxy-?-(benzyl-tbutylamino)-
acetophenone which was recrystallized from acetone/ether. If the product is crystallized from different organic solvents, the melting point will
vary with the type and amount of solvent of crystallization, but the product
can be used directly for hydrogenation.
15 g of 3,5-dibenzyloxy-?-(benzyl-t-butylamino)-acetophenone hydrogen
sulfate in 200 ml of glacial acetic acid were hydrogenated in a Parr pressure
reaction apparatus in the presence of 1.5 g of 10% palladium charcoal at
50°C and 5 atmospheres pressure. The reaction time was 5 hours. The
catalyst was filtered off, the filtrate was evaporated to dryness and the
hydrogen sulfate of 1-(3',5'-dihydroxyphenyl)-2-(t-butylamino)-ethanol was
received. This compound is hygroscopic, but it can be transformed into a
nonhygroscopic sulfate in the following manner.
The hydrogen sulfate was dissolved in water and the pH of the solution was
adjusted to 5.6 (pH-meter) with 0.1 N sodium hydroxide solution. The water
solution was evaporated to dryness and the residue dried with absolute
ethanol/benzene and once more evaporated to dryness. The remaining crystal
mixture was extracted in a Soxhlet extraction apparatus with absolute
methanol. From the methanol phase the sulfate of 1-(3',5'-dihydroxyphenyl)-
2-(t-butylamino)-ethanol crystallized. Melting point 246°C to 248°C.
brand name
Terbutaline is INN and BAN.
Therapeutic Function
Bronchodilator
Clinical Use
Beta2
-adrenoceptor agonist:
Reversible airways obstruction
Veterinary Drugs and Treatments
Terbutaline is used as a bronchodilating agent in the adjunctive
treatment of cardiopulmonary diseases (including tracheobronchitis,
collapsing trachea, pulmonary edema, and allergic bronchitis)
in small animals. It may be of some benefit in treating bradyarrhythmias
in dogs and cats.
Terbutaline has been used occasionally in horses for its bronchodilating
effects, but adverse effects, short duration of activity after
IV administration and poor oral absorption have limited its use.
It has been shown to be useful as a diagnostic agent to diagnose
anhidrosis in horses after intradermal injection.
Oral and intravenous terbutaline has been used successfully in
humans for the inhibition of premature labor clinical signs.
Drug interactions
Potentially hazardous interactions with other drugs
Effect may be diminished by beta-blockers.
Theophylline: increased risk of hypokalaemia.
Metabolism
Terbutaline undergoes extensive first-pass metabolism by sulphate (and some glucuronide) conjugation in the liver and the gut wall. It is excreted in the urine and faeces partly as the inactive sulphate conjugate and partly as unchanged terbutaline, the ratio depending upon the route by which it is given.
Properties of TERBUTALINE SULFATE
| Melting point: | 246-248°C |
| Density | 1.1840 (rough estimate) |
| refractive index | 1.6900 (estimate) |
| storage temp. | 2-8°C |
| solubility | H2O: soluble100mg/mL, clear to slightly hazy, colorless to yellow |
| form | neat |
| pka | pKa1 8.8, pKa2 10.1, pKa3 11.2(at 25℃) |
| form | Solid |
| color | White to Off-White |
| InChI | InChI=1S/2C12H19NO3.H2O4S/c2*1-12(2,3)13-7-11(16)8-4-9(14)6-10(15)5-8;1-5(2,3)4/h2*4-6,11,13-16H,7H2,1-3H3;(H2,1,2,3,4) |
Safety information for TERBUTALINE SULFATE
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H361:Reproductive toxicity |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for TERBUTALINE SULFATE
| InChIKey | KFVSLSTULZVNPG-UHFFFAOYSA-N |
| SMILES | C1(=CC(=CC(O)=C1)O)C(O)CNC(C)(C)C.C1(=CC(=CC(O)=C1)O)C(O)CNC(C)(C)C.S(=O)(=O)(O)O |
TERBUTALINE SULFATE manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 Terbutaline Sulphate 99%View Details
Terbutaline Sulphate 99%View Details
23031-32-5 -
 Terbutaline Sulphate USP / BP /EP/IP 23031-32-5 99%View Details
Terbutaline Sulphate USP / BP /EP/IP 23031-32-5 99%View Details
23031-32-5 -
 23031-32-5 Terbutaline sulfate 98%View Details
23031-32-5 Terbutaline sulfate 98%View Details
23031-32-5 -
 23031-32-5 Terbutaline sulfate 98%View Details
23031-32-5 Terbutaline sulfate 98%View Details
23031-32-5 -
 Terbutaline Sulfate CAS 23031-32-5View Details
Terbutaline Sulfate CAS 23031-32-5View Details
23031-32-5 -
 Terbutaline sulfate CAS 23031-32-5View Details
Terbutaline sulfate CAS 23031-32-5View Details
23031-32-5 -
 Terbutaline sulfate CAS 23031-32-5View Details
Terbutaline sulfate CAS 23031-32-5View Details
23031-32-5 -
 Terbutaline Sulphate Powder, 50kg BagView Details
Terbutaline Sulphate Powder, 50kg BagView Details
23031-25-6
