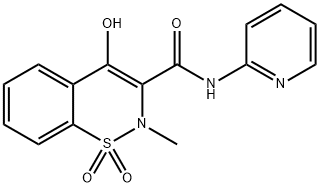
Tenoxicam
Synonym(s):4-Hydroxy-2-methyl-N-2-pyridinyl-2H-thieno(2,3-e)-1,2-thiazine-3-carboxamide 1,1-dioxide
- CAS NO.:59804-37-4
- Empirical Formula: C13H11N3O4S2
- Molecular Weight: 337.37
- MDL number: MFCD00083502
- EINECS: 620-500-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33

What is Tenoxicam?
Absorption
Oral absorption of tenoxicam is rapid and complete (absolute bioavailability 100%).
Description
Tenoxicam is a non-steroidal antiinflammatory agent with a profile similar to related piroxicam and now withdrawn isoxicam (55). It is useful in the treatment of rheumatoid arthritis, osteoarthritis and related disorders.
Chemical properties
Yellow Crystalline Powder
Originator
Hoffmann-La Roche (Switzerland)
The Uses of Tenoxicam
Tenoxicam has been used:
- as a non-steroidal anti-inflammatory agent (NSAID) to study its effects on root gravitropism in Arabidopsis thaliana
- as a standard in microanalysis of NSAIDs by spectrophotometry
- to test its effect on surface potential andmembrane fluidity modification in phosphoglyceride monolayers
Background
Tenoxicam, an antiinflammatory agent with analgesic and antipyretic properties, is used to treat osteoarthritis and control acute pain.
What are the applications of Application
Tenoxicam is a nonsteroidal, anti-inflammatory, analgesic agent
Indications
For the treatment of rheumatoid arthritis, osteoarthritis, backache, and pain.
Definition
ChEBI: Tenoxicam is a thienothiazine-derived monocarboxylic acid amide obtained by formal condensation of the carboxy group of 4-hydroxy-2-methylthieno[2,3-e][1,2]thiazine-3-carboxylic acid 1,1-dioxide with the amino group of 2-aminopyridine. Used for the treatment of pain and inflammation in osteoarthritis and rheumatoid arthritis. It is also indicated for short term treatment of acute musculoskeletal disorders including strains, sprains and other soft-tissue injuries. It has a role as a non-steroidal anti-inflammatory drug, a non-narcotic analgesic, an antipyretic and an EC 1.14.99.1 (prostaglandin-endoperoxide synthase) inhibitor. It is a heteroaryl hydroxy compound, a monocarboxylic acid amide, a member of pyridines and a thienothiazine.
brand name
Tilcotil
Biochem/physiol Actions
Tenoxicam (TX) possesses antipyretic?and analgesic effects. It elicits radical scavenging activity and has the potential to treat enkylosing spondylitis, extra-articular diseases, acute gout, and rheumatic diseases. It is also effective in treating primary dysmenorrhea, postpartum uterine contraction pain, and post-operation backaches. TX is capable of inhibiting prostaglandin synthesis.
Pharmacokinetics
Tenoxicam, an antiinflammatory agent with analgesic and antipyretic properties, is used to treat osteoarthritis and control acute pain.
Clinical Use
NSAID and analgesic
Synthesis
The reaction of methyl 3-hydroxythiophene- 2-carboxylate with PCl5 in refluxing CCl4 gives 3-chlorothiophene-2-carboxylic acid, which by treatment with NaHSO3 and Cu in aqueous alkaline solution at 143 ?C in a pressure vessel is converted into 3- sulfothiophene-2-carboxylic acid. Its esterification with refluxing methanol affords methyl- 3-sulfothiophene-2-carboxylate, which by reaction with refluxing SOCl2 yields methyl- 3-chlorosulfonylthiophene-2-carboxylate. The following condensation with sarcosine ethyl ester in hot CHCl3 gives 3-(N-ethoxycarbonylmethyl- N-methylsulfamoyl)thiophene-2-carboxylate, which is cyclized by treatment with sodium methoxide in refluxing methanol affording 3-ethoxycarbonyl-4-hydroxy-2-methyl-2Hthieno--1,2-thiazine 1,1-dioxide. Finally this compound is condensed with 2-aminopyridine in refluxing toluene
Drug interactions
Potentially hazardous interactions with other drugs
ACE inhibitors and angiotensin-II antagonists:
antagonism of hypotensive effect; increased risk of
nephrotoxicity and hyperkalaemia.
Analgesics: avoid concomitant use of 2 or more
NSAIDs, including aspirin (increased side effects);
avoid with ketorolac (increased risk of side effects
and haemorrhage).
Antibacterials: possibly increased risk of convulsions
with quinolones.
Anticoagulants: effects of coumarins and
phenindione enhanced; possibly increased risk of
bleeding with heparins, dabigatran and edoxaban -
avoid long term use with edoxaban.
Antidepressants: increased risk of bleeding with
SSRIs and venlaflaxine.
Antidiabetic agents: effects of sulphonylureas
enhanced.
Antiepileptics: possibly increased phenytoin
concentration.
Antivirals: increased risk of haematological toxicity
with zidovudine; concentration possibly increased by
ritonavir.
Ciclosporin: may potentiate nephrotoxicity.
Cytotoxics: reduced excretion of methotrexate;
increased risk of bleeding with erlotinib.
Diuretics: increased risk of nephrotoxicity;
antagonism of diuretic effect; hyperkalaemia with
potassium-sparing diuretics.
Lithium: excretion decreased.
Pentoxifylline: increased risk of bleeding.
Tacrolimus: increased risk of nephrotoxicity.
Metabolism
Tenoxicam is metabolized in the liver to several pharmacologically inactive metabolites (mainly 5'-hydroxy-tenoxicam).
Metabolism
Metabolised in the liver via cytochrome P450 2C9 to
several pharmacologically inactive metabolites (mainly
5'-hydroxy-tenoxicam).
Metabolites are excreted mainly in the urine; there is
some biliary excretion of glucuronide conjugates of the
metabolites.
Properties of Tenoxicam
| Melting point: | 209-2130C (dec) |
| Density | 1.4737 (rough estimate) |
| refractive index | 1.6390 (estimate) |
| storage temp. | 2-8°C |
| solubility | Practically insoluble in water, sparingly soluble in methylene chloride, very slightly soluble in anhydrous ethanol. It dissolves in solutions of acids and alkalis. |
| form | neat |
| pka | pKa1 5.3, pKa2 1.1(at 25℃) |
| form | Solid |
| color | White to Yellow to Green |
| Water Solubility | 61.9mg/L(32 ºC) |
| CAS DataBase Reference | 59804-37-4 |
Safety information for Tenoxicam
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Tenoxicam
Tenoxicam manufacturer
PROTECH TELELINKS
BDR Pharmaceuticals International Pvt Ltd
Clickchem Research LLP
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![METHYL 4-HYDROXY-2H-THIENO[2,3-E]-1,2-THIAZINE-3-CARBOXYLATE-1,1-DIOXIDE](https://img.chemicalbook.in/CAS/GIF/98827-44-2.gif)
![METHYL 2-METHYL-4-HYDROXY-2H-THIENO[2,3-E]-1,2-THIAZINE-3-CARBOXYLATE-1,1-DIOXIDE](https://img.chemicalbook.in/CAS/GIF/59804-25-0.gif)

You may like
-
 59804-37-4 Tenoxicam 98%View Details
59804-37-4 Tenoxicam 98%View Details
59804-37-4 -
 59804-37-4 98%View Details
59804-37-4 98%View Details
59804-37-4 -
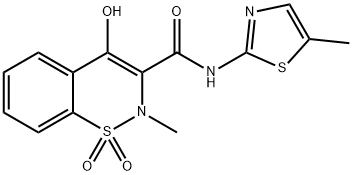
 Deschloro analog of lornoxicam/tenoxicam 90 % AboveView Details
Deschloro analog of lornoxicam/tenoxicam 90 % AboveView Details
59804-37-4 -
 Tenoxicam CAS 59804-37-4View Details
Tenoxicam CAS 59804-37-4View Details
59804-37-4 -
 Tenoxicam 98.00% CAS 59804-37-4View Details
Tenoxicam 98.00% CAS 59804-37-4View Details
59804-37-4 -
 Tenoxicam CAS 59804-37-4View Details
Tenoxicam CAS 59804-37-4View Details
59804-37-4 -
 Tenoxicam degradation impurity standard CAS 59804-37-4View Details
Tenoxicam degradation impurity standard CAS 59804-37-4View Details
59804-37-4 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1