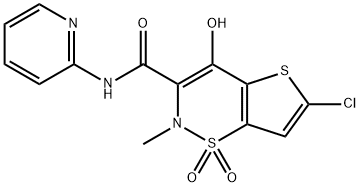
Lornoxicam
Synonym(s):6-Chloro-4-hydroxy-2-methyl-N-2-pyridinyl-2H-thieno[2,3-e]-1,2-thiazine-3-carboxamide 1,1-dioxide;Chlortenoxicam;Ro 13-9297;TS 110
- CAS NO.:70374-39-9
- Empirical Formula: C13H10ClN3O4S2
- Molecular Weight: 371.82
- MDL number: MFCD00866163
- EINECS: 630-354-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
What is Lornoxicam?
Absorption
Lornoxicam is absorbed rapidly and almost completely from the GI tract (90-100%).
Description
Lornoxicam is a nonselective NSAID for oral and intravenous administration. It has been available for human use since two decades and there is a growing body of evidence supporting its efficacy and tolerability for management of acute pain.
Xefo, a member of the oxicam family of nonsteroidal antiinflammatory agents, was launched in Denmark for mild to moderate pain and inflammation. A seven step synthesis, beginning with 2,5-dichlorothiophene, provides access to this compound. It inhibits prostaglandin synthesis at the level of cyclooxygenase (Cox1 :Cox2 = 0.6) but does not inhibit 5-lipoxygenase. Xefo did not increase serum levels of pepsinogen I (an indicator of gastric mucosal status) and readily penetrates perivascular interstitial spaces including synovial fluid. It is as effective as morphine, meperidine and tramadol in relieving post operative pain and as efficient as other NSAlDs in relieving the symptoms of osteoarthritis and rheumatoid arthritis. It is 100 times more potent than Tenoxicam in inhibiting PGD2 and more active than indomethacin (6x) or piroxicam (10x) in preventing arachadonic acid influenced lethality in mice. Xefo has inhibitor effects on spinal nocicceptive processing presumably via release of endogenous opiods and evidence suggests it is good for migraine.
Description
Lornoxicam is a COX inhibitor and non-steroidal anti-inflammatory drug (NSAID) with anti-inflammatory and analgesic properties. It inhibits production of thromboxane B2 (TXB2; ) from arachidonic acid (Item Nos. 90010 | 90010.1 | 10006607) in HEL human erythroleukemic cells (IC50 = 3 nM), which endogenously express COX-1, as well as inhibits LPS-induced formation of prostaglandin F1α (PGF1α; ) from arachidonic acid in Mono-Mac-6 cells (IC50 = 8 nM), which endogenously express COX-2. Lornoxicam reduces LPS-induced production of nitric oxide and IL-6 in cell-based assays with IC50 values of 65 and 54 μM, respectively. It reduces carrageenan-induced paw edema in rats when administered intravenously at doses ranging from 0.1 to 9 mg/kg. Formulations containing lornoxicam have been used in the management of postoperative pain.
Chemical properties
Crystalline Solid
Originator
Nycomed Amersham (Norway)
The Uses of Lornoxicam
Lornoxicam belongs to the oxicam class. It has anti-inflammatory and antipyretic properties. Lornoxicam prevents the synthesis of prostaglandin (PG) by inhibiting cyclo-oxygenase. It is used to relieve various types of symptoms associated with osteoarthritis, rheumatoid arthritis, ankylosing spondylitis, acute sciatica and low back pain.Lornoxicam is approved for use in Japan.
Lornoxicam has been used as a drug in melanin binding study with cassette dosing and rapid equilibrium dialysis inserts. Lornoxicam differs from other oxicam compounds in its potent inhibition of prostaglandin biosynthesis, a property that explains the particularly pronounced efficacy of the drug.
The Uses of Lornoxicam
Cyclooxygenase inhibitor; structurally similar to tenoxicam. Anti-inflammatory; analgesic
The Uses of Lornoxicam
keratolytic, antiacne, antineoplastic
The Uses of Lornoxicam
For the treatment of acute mild to moderate pain, as well as pain and inflammation of the joints caused by certain types of rheumatic diseases.
Indications
For the treatment of acute mild to moderate pain, as well as pain and inflammation of the joints caused by certain types of rheumatic diseases.
Background
Lornoxicam (chlortenoxicam) is a new nonsteroidal anti-inflammatory drug (NSAID) of the oxicam class with analgesic, anti-inflammatory and antipyretic properties. Lornoxicam differs from other oxicam compounds in its potent inhibition of prostaglandin biosynthesis, a property that explains the particularly pronounced efficacy of the drug. Lornoxicam is approved for use in Japan.
What are the applications of Application
Lornoxicam is a cyclooxygenase inhibitor
Definition
ChEBI: A thienothiazine-derived monocarboxylic acid amide obtained by formal condensation of the carboxy group of 6-chloro-4-hydroxy-2-methylthieno[2,3-e][1,2]thiazine-3-carboxylic acid 1,1-dioxide with the amino group of 2-aminopyridine. Used for th treatment of pain, primarily resulting from inflammatory diseases of the joints, osteoarthritis, surgery, sciatica and other inflammations.
Indications
Lornoxicam is a non-steroidal anti-inflammatory drug (NSAID) that is used as a painkiller (analgesic). A high level of pain relief is experienced by about 45% of those with moderate to severe postoperative dental pain after a single dose of lornoxicam 8 mg, compared to about 10% with placebo. This is comparable to the proportion experiencing the same level of pain relief with ibuprofen 200 to 400 mg. Adverse events were generally mild and did not differ from placebo in these singe dose studies. There were insufficient data to assess duration of action, but it is likely to be similar to ibuprofen 200 mg.
brand name
Xefo
Pharmacokinetics
Lornoxicam is a non-steroidal anti-inflammatory drug (NSAID) that belongs to the oxicam class. As with other NSAIDS, lornoxicam is a potent inhibitor of the cyclooxgenase enzymes, which are responsible for catalyzing the formation of prostaglandins (act as messenger molecules in the process of inflammation) and thromboxane from arachidonic acid. Unlike some NSAIDS, lornoxicam's inhibition of cyclooxygenase does not lead to an increase in leukotriene formation, meaning that arachidonic acid is not moved to the 5-lipoxygenase cascade, resulting in the minimization of the risk of adverse events.
in vitro
studies on intact human cells showed that lornoxicam intensively inhibit cox-1 and cox-2 with the lowest ic50 among a large panel of nsaids tested. similar findings were obtained in the whole blood for cox-1/-2. in addition lornoxicam suppressed no formation in a dose-dependently manner with an ic50 of 65 μm. [2]
in vivo
in vivo studies found that lornoxicam was as effective as comparative nsaids and that 8 mg lornoxicam was more effective than 10 mg morphine as a pain-reliever after oral surgery. orally administration of lornoxicam at 16-24 mg daily was more effective than tramadol at 300 mg daily in pain-alleviating after knee surgery. compared to naproxen, lornoxicam showed higher therapeutic potency and lower gastrointestinal toxicity. this was probably due to the short half-life of lornoxicam as compared to the other oxicams. [3]
Metabolism
Lornoxicam is metabolized completely by cyp 2C9 with the principal metabolite being 5'-hydroxy-lornoxicam and only negligible amounts of intact lornoxicam are excreted unchanged in the urine. Approximately 2/3 of the drug is eliminated via the liver and 1/3 via the kidneys in the active form.
References
[1]balfour ja, fitton a and barradell lb. lornoxicam, a review of its pharmacology and therapeutic potential in the management of painful and inflammatory conditions. drugs. 1996 apr; 51(4): 639-57.
[2]berg j, fellier h, christoph t, grarup j and stimmeder d. the analgesic nsaid lornoxicam inhibits cyclooxygenase (cox)-1/-2, inducible nitric oxide synthase (inos), and the formation of interleukin (il)-6 in vitro. inflamm res. 1999 jul; 48(7): 369-79.
[3]radhofer-welte s and rabasseda x. lornoxicam, a new potent nsaid with an improved tolerability profile. drugs today (barc). 2000 jan; 36(1): 55-76.
[4]sharma a, pingle a, and baliga vp. lornoxicam efficacy in acute pain (leap) trial. j indian med assoc. 2008 dec; 106(12): 811-3.
Properties of Lornoxicam
| Melting point: | 225-230°C (dec.) |
| Density | 1.742±0.06 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C |
| solubility | DMSO: >5mg/mL (warmed) |
| pka | pKa2 4.7(at 25℃) |
| form | powder |
| color | faint yellow to dark yellow |
| Merck | 14,5582 |
| CAS DataBase Reference | 70374-39-9(CAS DataBase Reference) |
Safety information for Lornoxicam
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H300:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Lornoxicam
| InChIKey | WLHQHAUOOXYABV-UHFFFAOYSA-N |
Lornoxicam manufacturer
Prayosha Health Care Pvt Ltd
Alex Pharmachem Pvt. Ltd
Paradise Healthcare
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran





You may like
-
 Lornoxicam 99%View Details
Lornoxicam 99%View Details -
 Lornoxicam 99%View Details
Lornoxicam 99%View Details -
 Lornoxicam 99%View Details
Lornoxicam 99%View Details -
 Lornoxicam, 98% CAS 70374-39-9View Details
Lornoxicam, 98% CAS 70374-39-9View Details
70374-39-9 -
 70374-39-9 98%View Details
70374-39-9 98%View Details
70374-39-9 -
 Lornoxicam 99%View Details
Lornoxicam 99%View Details
70374-39-9 -
 Lornoxicam 95% CAS 70374-39-9View Details
Lornoxicam 95% CAS 70374-39-9View Details
70374-39-9 -
 Lornoxicam CAS 70374-39-9View Details
Lornoxicam CAS 70374-39-9View Details
70374-39-9