Temoporfin
- CAS NO.:122341-38-2
- Empirical Formula: C44H32N4O4
- Molecular Weight: 680.75
- MDL number: MFCD00867835
- EINECS: 624-374-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
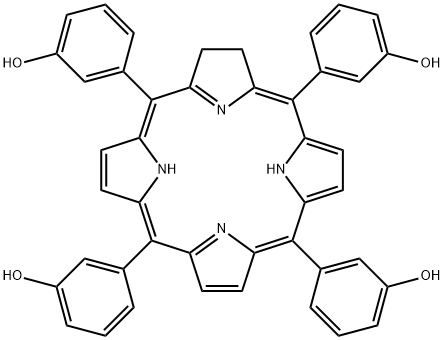
What is Temoporfin?
Absorption
Tmax is 2-4 h after intravenous administration . Plasma concentration initially decreases rapidly then slowly rises to reach peak serum concentration .
Toxicity
Mice and rats experienced swelling and darkening of exposed tissue at single dosages of >0.85 mg/kg under normal lighting . Systemic toxicity presented as reduced red blood cell and platelet counts and increased white blood cell counts and liver and spleen weights. Skin inflammation, pycnotic spermatocytes and increased extramedullary haematopoiesis in spleen and the lymph nodes was also observed. Under low-light conditions mild phototoxicity was observed only at high doses.
Severe phototoxicity has been seen in rats with repeated doses of up to 1 mg/kg/day under normal lighting . This effect is less severe under low-light conditions. Two weeks of repeated doses of 0.5-0.6 mg/kg/day resulted in inflammation of the injection site and skin in rats. At 0.3 mg/kg/day under low-light in rats, the only effect seen was an increase in white blood cell counts.
In beagle dogs recieving repeated doses of up to 3mg/kg/day under low-light conditions, reddening of the skin and injection site inflammation was seen . Serious injection site damage was observed.
Description
Temoporphin, a second generation photosensitizer, was launched in UK for the photodynamic therapy (PDT) of advanced head and neck cancers. This porphyrin derivative can be synthesized from pyrrole and 3-hydroxybenzaldehyde. The pharmacological activity is initiated, 4 days after intravenous injection, by laser-light photoactivation of temoporphin, that has selectively accumulated in cancer tissues. The resulting generation of highly reactive oxygen species leads to malignant cells death thereby inducing tumor necrosis up to a depth of 15 mm. An advantage of temoporphin over other photosensitizing agents is its extreme sensitivity to wavelengths of light that penetrate tissues, resulting in lower light/dose and irradiation time. PDT with intravenous temoporphin has produced relatively high complete and partial response rates in head and neck cancers, with higher response rates generally observed with higher light dose. Temoporphin is well-tolerated and does not preclude surgery/radiotherapy as a later option. Adverse effects were photosensitivity and pain at the injection site.
Originator
Quanta Nova (UK)
The Uses of Temoporfin
Temoporfin is a synthetic chlorin with light-activated actions. When administered systemically, temoporfin accumulates in tumor cells. When stimulated with light (650-652 nm) in the presence of oxygen, reactive oxygen species are generated, leading to necrosis within the tumor. Different approaches to using photodynamic therapy with temoporfin in palliative care are currently of interest.
The Uses of Temoporfin
Temoporfin is a synthetic chlorin with light-activated actions. It accumulates in tumor cells and through light activation and oxygen radical formation, it leads to necrosis and cell death.
Background
Temoporfin is a photosensitizing agent used in the treatment of squamous cell carcinoma of the head and neck . It was first authorized for market by the European Medicines Agency in October 2001. It is currently available under the brand name Foscan.
Indications
For use in the treatment of patients with advanced squamous cell carcinoma of the head and neck failing standard therapies and who are unsuitable for radiotherapy, surgery, or systemic chemotherapy .
Definition
ChEBI: Temoporfin is a member of chlorins. It has a role as a photosensitizing agent.
brand name
Foscan
Pharmacokinetics
Temoporfin is a photosensitizing agent . It enters cancer cells and is activated via light to produce reactive species which destroy the cell.
Metabolism
The exact metabolic reactions Temoporfin undergoes are unknown. The drug metabolites have been identified as conjugates but specific information is unavailable.
Properties of Temoporfin
| Melting point: | >250 °C |
| Density | 1.386±0.06 g/cm3(Predicted) |
| storage temp. | Amber Vial, -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| color | Purple |
Safety information for Temoporfin
Computed Descriptors for Temoporfin
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 3-(Hydroxymethyl)benzoate N-Boc-2-chloroethylamine 1-Bromo-2-methoxy-3-nitrobenzene N-Methyl-3-cyclopenten-1-amine 2-Bromo-3-hydroxybenzaldehyde 1H-indazole-5-carboxamideRelated products of tetrahydrofuran


You may like
-
 Temoporfin CAS 122341-38-2View Details
Temoporfin CAS 122341-38-2View Details
122341-38-2 -
 7441-43-2 98%View Details
7441-43-2 98%View Details
7441-43-2 -
 1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 -
 4-bromo-3,5-dimethylbenzenesulfonyl chloride 1581266-79-6 98%View Details
4-bromo-3,5-dimethylbenzenesulfonyl chloride 1581266-79-6 98%View Details
1581266-79-6 -
 2490430-37-8 98%View Details
2490430-37-8 98%View Details
2490430-37-8 -
 N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
5434-30-0 -
 124371-59-1 98%View Details
124371-59-1 98%View Details
124371-59-1 -
 53857-52-2 98%View Details
53857-52-2 98%View Details
53857-52-2