Phthalocyanine
- CAS NO.:574-93-6
- Empirical Formula: C32H18N8
- Molecular Weight: 514.54
- MDL number: MFCD00005085
- EINECS: 209-378-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
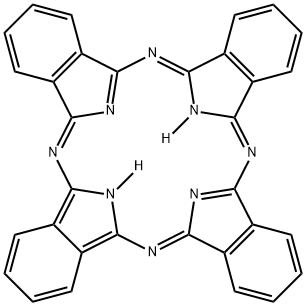
What is Phthalocyanine?
Description
Phthalocyanine is an aromatic, intensely blue-green heterocyclic compound. Its structure is similar to that of porphine (the framework of molecules such as chlorophyll) with four fused benzene rings. It forms dye complexes with most metals in the periodic table, notably copper. A. Braun and J. Tcherniac reported the free molecule in 1907, but didn’t establish its structure. The copper form was made accidentally in 1927 when researchers were trying to make phthalonitrile.
Phthalocyanine dyes have been widely used for decades. But the molecule’s electronic properties make it potentially useful in the hi-tech world. Japanese researchers are devising ways to slow the tautomerization rate of phthalocyanine so that the molecule can exist in two electronically distinct states, corresponding to the binary system that has been the basis of computer technology. This development would give rise to “molecular memory” that could eventually be used in extremely fast computers. Phthalocyanine is also very compact: It may be able to store ≈13 terabytes of data on a 1-cm2?chip.
Chemical properties
Blue to purple powder
The Uses of Phthalocyanine
Phthalocyanine is a common macrocylic blue-green dye able to form coordination complexes with many elements on the periodic table.
What are the applications of Application
Phthalocyanine is a common macrocylic blue-green dye
Definition
ChEBI: A tetrapyrrole fundamental parent that consists of four isoindole-type units, with the connecting carbon atoms in the macrocycle replaced by nitrogen.
Definition
phthalocyanine: A synthetic compoundhaving molecules with fourisoindole rings linked by –N= bridges.The structure is similar to that of theporphyrins. It can form complexeswith central metal ions. Copperphthalocyanines are used as dyes.
Flammability and Explosibility
Not classified
Agricultural Uses
Phthalocyanine is a group of benzoporphyrins which
have strong pigmenting power, forming a family of dyes.
The basic structure of the molecule comprises four
isoindole groups (C6H4,)C2N, joined by four nitrogen
atoms. Four commercially important modifications are:
(a) metal free phthalocyanine (C6H4C2N)4, having a bluegreen
color,(b copper phthalocyanine in which a copper
atom is held by secondary valences of the isoindole
nitrogen atoms, (c) chlorinated copper phthalocyanine,
green, in which 15 to 16 hydrogen atoms are replaced by
chlorine, and (d) sulphonated copper phthalocyanine,
water-soluble and green, in which two hydrogens are
replaced by sulphonic acid groups. It is used in decorative
enamels and automotive finishes; chlorophyll and
haems have basic phthalocyanine structures in their
molecules.
Purification Methods
Purify phthalocyanine by sublimation (two to three times) in an argon flow at 300-400Pa; and similarly for the Cu(II), Ni(II), Pb(II), VO(II) and Zn(II) phthalocyanine complexes. [Beilstein 26 III/IV 4255.]
Properties of Phthalocyanine
| Melting point: | >300 °C (dec.)(lit.) |
| Boiling point: | 590.15°C (rough estimate) |
| Density | 1.6931 (rough estimate) |
| refractive index | 1.9320 (estimate) |
| storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C |
| Colour Index | 74100 |
| form | Powder |
| color | blue black |
| Water Solubility | Insoluble in water. |
| BRN | 378323 |
| EPA Substance Registry System | 29H,31H-Phthalocyanine (574-93-6) |
Safety information for Phthalocyanine
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Phthalocyanine
Phthalocyanine manufacturer
Dr. Silviu Pharmachem Pvt., Ltd.
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![copper,[n,n’,n’’-tris[3-(dimethylamino)propyl]-29h,31h-phthalocyanine-c,c,c-t](https://img.chemicalbook.in/CAS/GIF/33481-16-2.gif)
![Cuprate(4-),[29H,31H-phthalocyanine-C,C,C,C-tetrasulfonato(6-)-N29,N30,N31,N32]-,tetrasodiu](https://img.chemicalbook.in/CAS/GIF/27360-85-6.gif)

You may like
-
 574-93-6 29H,31H-Phthalocyanine 98%View Details
574-93-6 29H,31H-Phthalocyanine 98%View Details
574-93-6 -
 Phthalocyanine CAS 574-93-6View Details
Phthalocyanine CAS 574-93-6View Details
574-93-6 -
 Phthalocyanine CAS 574-93-6View Details
Phthalocyanine CAS 574-93-6View Details
574-93-6 -
 Phthalocyanine (purified by sublimation) CAS 574-93-6View Details
Phthalocyanine (purified by sublimation) CAS 574-93-6View Details
574-93-6 -
 Phthalocyanine CAS 574-93-6View Details
Phthalocyanine CAS 574-93-6View Details
574-93-6 -
 Pigment Blue 16 98.00% CAS 574-93-6View Details
Pigment Blue 16 98.00% CAS 574-93-6View Details
574-93-6 -
 29H,31H-Phthalocyanine CAS 574-93-6View Details
29H,31H-Phthalocyanine CAS 574-93-6View Details
574-93-6 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1