TELLURIC ACID
Synonym(s):Orthotelluric acid
- CAS NO.:7803-68-1
- Empirical Formula: H6O6Te
- Molecular Weight: 229.64404
- MDL number: MFCD00011609
- EINECS: 232-267-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
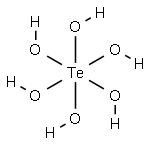
What is TELLURIC ACID?
Description
Telluric acid, H6TeO6, is obtained in the form of its salts
when tellurium is fused with potassium nitrate plus
carbonate, or by the oxidizing action of chlorine on a tellurite
in alkaline solution. The free acid may be obtained
by decomposing the barium salt with sulfuric acid and
concentrating the solution.
where tellurium is in an octahedral coordination.
Telluric acid, Te(OH)6, has the CAS number of 7803-68-
1 and a molecular weight of 229.6425 g/mol. It is a white,
monoclinic crystal with a density of 3.07 g/cm3. Its
melting point is 136°C and its solubility in water is
50.1 g/100 ml.
It is also formed when tellurium dioxide is oxidized
by hydrogen peroxide in caustic solution (A. Gutbier,
Zest. Anorg. Chem., 1904, 40, p. 260), and perhaps best
of all by oxidizing tellurium with a mixture of nitric
and chromic acids. It crystallizes as prisms, which lose
their water of hydration at 160°C. The tellurates of the
alkaline earth metals are more or less soluble in water,
those of the other metals being very sparingly or almost
insoluble in water. Some tellurates exist in two forms,
a colorless form soluble in water and acids, and a yellow
form insoluble in water and acids.
Chemical properties
White, heavy crystals. Soluble in hot water and alkalies; slightly soluble in cold water.
Physical properties
White crystals; dimorphic solid; exists in both cubic and monoclinic crystalline forms; density 3.07g/cm3; melts at 136°C; tends to polymerize (similar to stannic acid); forms polymetatelluric acid (H2TeO4)n on strong heating; soluble in water, about 33g/100 mL at 30°C; the solubility decreases as the molecule polymerizes and becomes colloidal; a very weak dibasic acid, pKa1 7.68 and pKa2 11.0 at 18°C; soluble in dilute nitric acid and alkalies.
The Uses of TELLURIC ACID
Chemical reagent.
Preparation
Telluric acid can be prepared by reducing barium tellurate with sulfuric acid: BaTeO4 + H2SO4 + 2H2O → H6TeO6 + BaSO4 Also, telluric acid can be prepared by oxidation of tellurium or tellurium dioxide with a strong oxidizing agent such as hydrogen peroxide, sodium peroxide, chromic acid, or potassium permanganate in nitric acid. Molecular equations for overall reactions are shown below: Te + 3H2O2 → H6TeO6 TeO2 + H2O2 + 2H2O → H6TeO6 Te + 2CrO3 + 3H2O → H6TeO6 + Cr2O3
At cold temperatures at about 1°C, telluric acid crystallizes as tetrahydrate.
Definition
ChEBI: Orthotelluric acid is a tellurium oxoacid. It is a conjugate acid of an orthotellurate(1-).
General Description
Telluric acids exist in both cubic and monoclinic crystalline forms.1 It may be prepared by treating tellurium and tellurous acid with strong oxidants.
Hazard
As for tellurium.
Safety Profile
Poison by ingestion and intravenous routes. When heated to decomposition it emits toxic fumes of Te. See also TELLURIUM COMPOUNDS.
Purification Methods
Crystallise it once from nitric acid, then repeatedly from hot water (0.4mL/g). [Fehér in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I pp 451-453 1963.]
Properties of TELLURIC ACID
| Melting point: | 136°C |
| Boiling point: | 0℃ |
| Density | 3.068; d 3.163 |
| solubility | H2O: 0.1 g/mL, clear, colorless |
| pka | 7.7(at 25℃) |
| form | white monoclinic crystals |
| color | White to Almost white |
| Water Solubility | g H2TeO6/100g H2O: 16.2 (0°C), 41.6 (20°C), 155 (100°C) [LAN05]; tends to polymerize; soluble dilute HNO3 [MER06] |
| λmax | 275nm(H2O)(lit.) |
| Merck | 14,9120 |
| Solubility Product Constant (Ksp) | pKsp: 53.52 |
| EPA Substance Registry System | Telluric acid (H6TeO6) (7803-68-1) |
Safety information for TELLURIC ACID
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H332:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. |
Computed Descriptors for TELLURIC ACID
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 Telluric Acid CAS 7803-68-1View Details
Telluric Acid CAS 7803-68-1View Details
7803-68-1 -
 Telluric acid CAS 7803-68-1View Details
Telluric acid CAS 7803-68-1View Details
7803-68-1 -
 Telluric acid CAS 7803-68-1View Details
Telluric acid CAS 7803-68-1View Details
7803-68-1 -
 Telluric acid CAS 7803-68-1View Details
Telluric acid CAS 7803-68-1View Details
7803-68-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1