SODIUM TELLURITE
Synonym(s):Disodium trioxotellurate
- CAS NO.:10102-20-2
- Empirical Formula: Na2O3Te
- Molecular Weight: 221.57774
- MDL number: MFCD00036141
- EINECS: 233-268-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
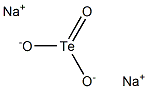
What is SODIUM TELLURITE?
Chemical properties
White powder. Soluble in water.
Chemical properties
Sodium tellurite is a white crystalline solid.
The Uses of SODIUM TELLURITE
Disodium Tellurite is an effector towards the thyroid hormone system due to it being of a similar group to selenium. Tellurium accumulates in the thyroid gland and influences the zinc thyroid level. I t is also damaging towards DNA in TK6 human lymphoblastoid cells.
The Uses of SODIUM TELLURITE
Sodium tellurite(IV) acts as an intermediate used in the extraction of the tellurium. It is used to make corrosion-resistant electroplated nickel. It is also used to add a bluish coating to iron, steel and aluminum. It serves as a growth medium for bacteria in microbiology.
General Description
White crystals. Used in bacteriology and medicine.
Air & Water Reactions
Water soluble.
Reactivity Profile
SODIUM TELLURITE is a weak reducing agent.
Hazard
Toxic by ingestion.
Health Hazard
The material is both an oral and dermal toxic hazard. The material is toxic by ingestion. Oral ingestion of tellurium compounds is generally regarded as extremely toxic. The probable oral lethal dose is 5-50 mg/kg or between 7 drops and 1 teaspoonful for a 70 kg (150 pound) person. Tellurium compounds are regarded as super toxic for skin exposures.
Fire Hazard
When heated to decomposition, SODIUM TELLURITE emits toxic fumes of tellurium and sodium monoxide.
Safety Profile
Human poison by ingestion and parenteral routes. Experimental poison by ingestion, intravenous, and intraperitoneal routes. Human systemic effects by ingestion: coma; dyspnea; nausea or vomiting. Human mutation data reported. When heated to decomposition it emits toxic fumes of Te and Na2O. See also TELLURIUM.
Potential Exposure
Used in bacteriology and medicine. Formerly used as pesticide.
Shipping
UN3284 Tellurium compound, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Incompatibilities
A weak reducing agent. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, cadmium, halogens. Reaction with oxidizers may generate heat and products that may be flammable, combustible, or otherwise reactive.
Properties of SODIUM TELLURITE
| solubility | Aqueous Acid (Slightly), Water (Slightly, Sonicated) |
| form | Powder |
| color | White |
| Water Solubility | Soluble in water. |
| Merck | 14,8685 |
| Exposure limits | ACGIH: TWA 0.1 mg/m3 NIOSH: IDLH 25 mg/m3; TWA 0.1 mg/m3 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 10102-20-2(CAS DataBase Reference) |
| EPA Substance Registry System | Sodium tellurite (10102-20-2) |
Safety information for SODIUM TELLURITE
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for SODIUM TELLURITE
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 Sodium tellurite(IV) CAS 10102-20-2View Details
Sodium tellurite(IV) CAS 10102-20-2View Details
10102-20-2 -
 10102-20-2 99%View Details
10102-20-2 99%View Details
10102-20-2 -
 Sodium tellurite, 98% CAS 10102-20-2View Details
Sodium tellurite, 98% CAS 10102-20-2View Details
10102-20-2 -
 SODIUM TELLURITE Extra Pure CAS 10102-20-2View Details
SODIUM TELLURITE Extra Pure CAS 10102-20-2View Details
10102-20-2 -
 Sodium tellurite CAS 10102-20-2View Details
Sodium tellurite CAS 10102-20-2View Details
10102-20-2 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
