SUGAMMADEX
- CAS NO.:343306-71-8
- Empirical Formula: C72H112O48S8
- Molecular Weight: 2002.15
- EINECS: 253-874-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-22 22:33:46
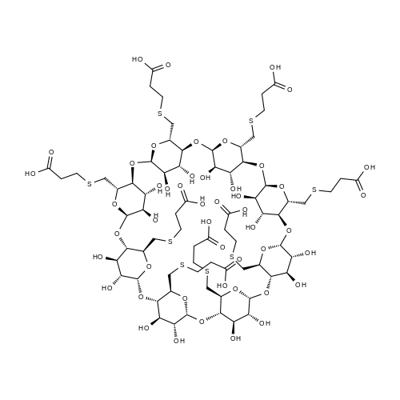
What is SUGAMMADEX?
Absorption
Sugammadex is administered intravenously.
Toxicity
Patients with severe renal impairment (with creatinine clearance below 30 mL/min) should avoid use of drug as their clearance of the drug is reduced and there is inconsistent evidence about its safety in this subset of patients.
Description
Sugammadex is a selective relaxant binding agent indicated for reversal of neuromuscular blockade induced by rocuronium bromide and vecuronium bromide during surgery in adults. Rocuronium bromide and vecuronium bromide are neuromuscular blocking medications that cause temporary paralysis and are especially useful for general anesthesia, ventilation, or tracheal intubation that patients may require for surgery. Sugammadex provides a new treatment option to reverse the effects of those medications and possibly help patients recover sooner post-surgery. Sugammadex (brand name Bridion) is marketed by Merck Sharp and Dohme, and was approved by the United States FDA on December 15, 2015.
The Uses of SUGAMMADEX
6A,6B,6C,6D,6E,6F,6G,6H-octakis-S-(2-carboxyethyl)-6A,6B,6C,6D,6E,6F,6G,6H-octathio-γ-Cyclodextrin, also known as Sugammadex, is used to reverse postoperative residual neuromuscular blockade.
The Uses of SUGAMMADEX
Reversal agent for neuromuscular blocking drugs.
Indications
Sugammadex is indicated for the reversal of neuromuscular blockade induced by rocuronium bromide or vecuronium bromide in adults and pediatric patients ≥2 years old who are undergoing surgery.
Background
Sugammadex is a selective relaxant binding agent indicated for reversal of neuromuscular blockade induced by rocuronium bromide and vecuronium bromide during surgery. Rocuronium bromide and vecuronium bromide are neuromuscular blocking medications that cause temporary paralysis and are especially useful for general anesthesia, ventilation, or tracheal intubation that patients may require for surgery. Sugammadex provides a new treatment option to reverse the effects of those medications and possibly help patients recover sooner post-surgery. Sugammadex (brand name Bridion) is marketed by Merck Sharp and Dohme, and was approved by the United States FDA on December 15, 2015.
Definition
ChEBI: An octasaccharide derivative that is gamma-cyclodextrin in which all eight primary hydroxy groups are replaced by 2-(carboxyethyl)sulfanyl groups. Used (as the octasodium salt) for reversal of neuromuscular blockade induced by rocuronium and vecuronium in adults undergoing surgery.
Synthesis
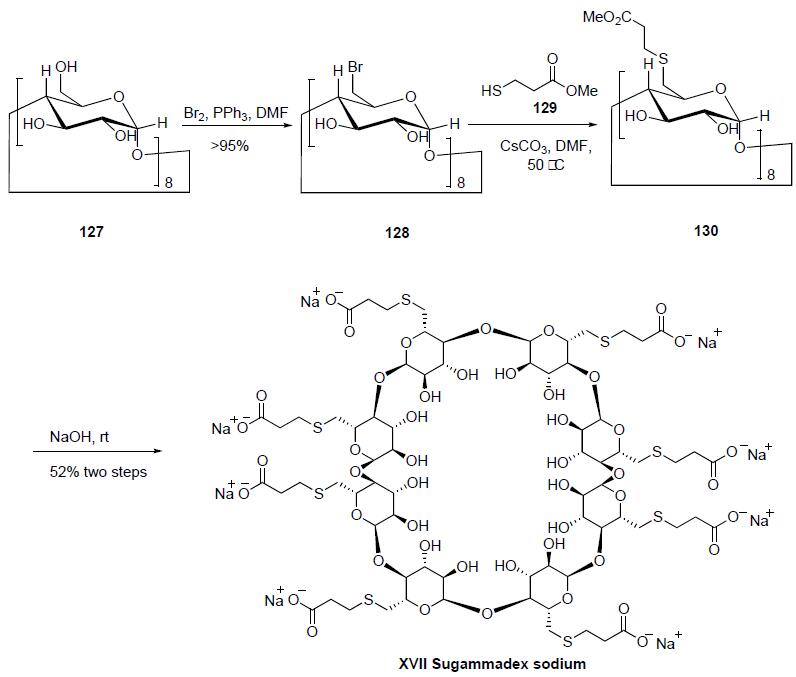
Bromination of |?-cyclodexdrin 127 with the Vilsmeier-Haack reagent prepared by reaction of bromine with triphenylphospine in DMF gave the per-6-bromo-|?-cyclodextrin 128 in 95-98% yield. Nucleophilic displacement of the bromines of 128 with methyl 3-mercaptopropionate (129) and cesium carbon-ate at 50??C in DMF gave 6-perdeoxy-6-per(2-methoxycarbonylethyl) thio-|?-cyclodextrin 130 as a white powder. Saponification of the esters of 130 was accomplished by reaction with aqueous sodium hydroxide solution to provide sugammadex (XVII) as a glassy solid in 52% yield for the 2 steps.
Metabolism
No metabolites of sugammadex were observed during clinical studies.
Properties of SUGAMMADEX
| Melting point: | >208°C (dec.) |
| Density | 1.559±0.06 g/cm3(Predicted) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | Aqueous Acid (Slightly), Water (Slightly, Sonicated) |
| form | Solid |
| pka | 3.42±0.10(Predicted) |
| color | White to Pale Beige |
| Stability: | Hygroscopic |
Safety information for SUGAMMADEX
Computed Descriptors for SUGAMMADEX
SUGAMMADEX manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 343306-71-8 SugaMMadex SodiuM 98%View Details
343306-71-8 SugaMMadex SodiuM 98%View Details
343306-71-8 -
 343306-71-8 98%View Details
343306-71-8 98%View Details
343306-71-8 -
 Sugammadex 98%View Details
Sugammadex 98%View Details -
 Sugammadex 343306-71-8 98%View Details
Sugammadex 343306-71-8 98%View Details
343306-71-8 -
 343306-71-8 Sugammadex 98%View Details
343306-71-8 Sugammadex 98%View Details
343306-71-8 -
 343306-71-8 98%View Details
343306-71-8 98%View Details
343306-71-8 -
 Sugammadex 96% CAS 343306-71-8View Details
Sugammadex 96% CAS 343306-71-8View Details
343306-71-8 -
 Sugammadex Sodium APIView Details
Sugammadex Sodium APIView Details
343306-71-8
