Sufentanil
- CAS NO.:56030-54-7
- Empirical Formula: C22H30N2O2S
- Molecular Weight: 386.55
- EINECS: 641-081-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-06-08 09:02:16
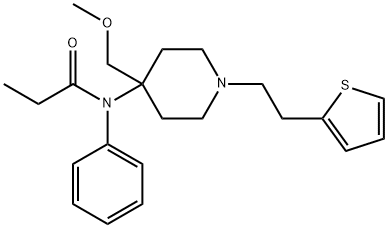
What is Sufentanil?
Absorption
Bioavailability of a single sublingual tablet was 52%, decreasing to 35% with repeat dosing .
After epidural administration of incremental doses totaling 5 to 40 mcg sufentanil during labor and delivery, maternal and neonatal sufentanil plasma concentrations were at or near the 0.05 to 0.1 ng/mL limit of detection, and were slightly higher in mothers than in their infants .
Toxicity
LD50: 18.7 mg/kg (IV in mice)
A Note on Respiratory Depression
Major, life-threatening, or fatal respiratory depression has been reported with the use of opioids, even in cases where it is used as recommended. Respiratory depression may lead to respiratory arrest and death if not diagnosed and treated appropriately. This drug should be administered only by persons specifically trained in the use of anesthetic drugs and the management of the respiratory effects of potent opioids, including respiration and cardiac resuscitation of patients in the age group being treated. This training must include the establishment and maintenance of a patent airway and assisted ventilation .
Carcinogenesis
Long-term studies in animals to evaluate the carcinogenic potential of sufentanil have not been conducted .
Mutagenesis
Sufentanil was not found to be genotoxic in the in vitro bacterial reverse mutation assay (Ames assay) or in the in vivo rat bone marrow micronucleous assay .
Reproductive Toxicity
Sufentanil caused embryolethality in rats and rabbits treated for 10-30 days during pregnancy with 2.5 times the maximum human dose by intravenous administration. The embryolethal effect was thought to be secondary to the toxicity for the mother animal model. No negative effects were noted in another study in rats that were treated with 20 times the maximum human dose in the period of organogenesis. The preclinical effects were only seen following administrations of levels significantly above the maximum human dose, which is therefore of minimal relevance for clinical use .
Pregnancy
May cause fetal harm
The Use in Lactation
Infants exposed to this drug through breast milk should be monitored for excess sedation and respiratory depression .
Description
Sufentanil is a narcotic analgesic with a greater potency and therapeutic ratio than its structural relative fentanyl. It appears to produce fewer cardiac effects and less respiratory depression than fentanyl, and thus is especially useful as an analgesic/anesthetic in open heart surgery.
Description
Sufentanil (Item No. 15917) is an analytical reference material categorized as an opioid. Sufentanil is regulated as a Schedule II compound in the United States. This product is intended for research and forensic applications.
Chemical properties
White or almost white powder.
Originator
Jaassen (Belgium)
The Uses of Sufentanil
Opioid receptor agonist; analgesic.
Background
Sufentanil is an opioid analgesic that is used as an adjunct in anesthesia, in balanced anesthesia, and as a primary anesthetic agent. It is administered by the intravenous, epidural and sublingual routes.
Also known as Dsuvia, the sublingual form is used for the management of acute pain in adults that is severe to warrant the use of an opioid analgesic in certified medically supervised healthcare settings, including hospitals, surgical centers, and emergency departments . Consideration may be made in the future for the use of the sublingual form in the US military in cases where analgesia is required immediately .
The sublingual form, manufactured by AcelRx Pharmaceuticals, Inc. (AcelRx), was approved on November 2, 2018 .
This route of administration is intended to be a simple, effective, non-invasive analgesic option to enable healthcare professionals to rapidly manage acute pain without difficult intravenous or epidural administration , .
Indications
The indications for this drug are as follows:
Definition
ChEBI: An anilide resulting from the formal condensation of the aryl amino group of 4-(methoxymethyl)-N-phenyl-1-[2-(2-thienyl)ethyl]piperidin-4-amine with propanoic acid.
Manufacturing Process
A mixture of 4.1 parts of N-[4-(methoxymethyl)-4-piperidinyl]-Nphenylpropanamide, 5.3 parts of sodium carbonate and 120 parts of 4- methyl-2-pentanone is stirred and refluxed with water-separator. Then there are added 4.1 parts of 2-thiopheneethanol methanesulfonate ester and stirring at reflux is continued for 18 hours. The reaction mixture is cooled, washed twice with water and evaporated. The oily residue is purified by columnchromatography over silica gel, using a mixture of trichloromethane and 5% of methanol as eluent. The first fraction is collected and the eluent is evaporated. The oily residue is converted into the hydrochloride salt in 2,2'- oxybispropane. The free base is liberated again in the conventional manner. After extraction with 2,2'-oxybispropane, the latter is dried, filtered and evaporated. The oily residue solidifies on triturating in petroleum-ether. The solid product is filtered off and crystallized from petroleum-ether at -20°C, yielding, after drying, N-[4-(methoxymethyl)-1-[2-(2-thienyl)ethyl]-4- piperidinyl]-N-phenylpropanamide; melting point 98.6°C.
brand name
SUFENTA
Therapeutic Function
Analgesic
Pharmacokinetics
Effect on the Central Nervous System (CNS)
In clinical settings, sufentanil exerts its principal pharmacologic effects on the central nervous system. Its primary therapeutic actions are analgesia and sedation. Sufentanil may increase pain tolerance and decrease the perception of pain. This drug depresses the respiratory centers, depresses the cough reflex, and constricts the pupils , . When used in balanced general anesthesia, sufentanil has been reported to be as much as 10 times as potent as fentanyl. When administered intravenously as a primary anesthetic agent with 100% oxygen, sufentanil is approximately 5 to 7 times as potent as fentanyl . High doses of intravenous sufentanil have been shown to cause muscle rigidity, likely as a result of an effect on the substantia nigra and the striate nucleus in the brain. Sleep-inducing (hypnotic) activity can be demonstrated by EEG alterations .
Effects on the Respiratory System
Sufentanil may cause respiratory depression .
Effects on the Cardiovascular System
Sufentanil causes peripheral vasodilation which may result in orthostatic hypotension or syncope. Bradycardia may also occur . Clinical signs or symptoms of histamine release and/or peripheral vasodilation may include pruritus, flushing, red eyes and sweating and/or orthostatic hypotension .
Effects on the Gastrointestinal Tract
Sufentanil causes a reduction in motility associated with an increase in smooth muscle tone in both the antrum of the stomach and duodenum. Digestion of food in the small intestine may be delayed and propulsive contractions are decreased. Propulsive peristaltic waves in the colon are decreased, while tone may be increased and lead to spasm, resulting in constipation. Other opioid-induced effects may include a reduction in biliary and pancreatic secretions, spasm of the sphincter of Oddi, as well as temporary elevations in serum amylase .
Metabolism
The liver and small intestine are the major sites of biotransformation . Sufentanil is rapidly metabolized to a number of inactive metabolites, with oxidative N- and O-dealkylation being the major routes of elimination .
Properties of Sufentanil
| Melting point: | 103-104℃ |
| Boiling point: | 493.1±40.0 °C(Predicted) |
| Density | 1.20 g/cm3 |
| Flash point: | 9℃ |
| storage temp. | 2-8°C |
| solubility | Practically insoluble in water, freely soluble in ethanol (96 per cent) and in methanol. |
| pka | 7.89±0.20(Predicted) |
| form | A neat solid |
| Water Solubility | 76mg/L(25 ºC) |
Safety information for Sufentanil
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H370:Specific target organ toxicity, single exposure |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P311:Call a POISON CENTER or doctor/physician. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Sufentanil
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4