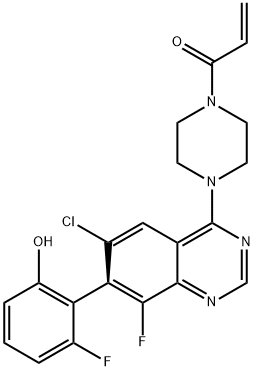
Sotorasib
- CAS NO.:2296729-00-3
- Empirical Formula: C30H30F2N6O3
- Molecular Weight: 560.61
- Update Date: 2024-11-18 17:01:59
What is Sotorasib?
Description
Sotorasib (AMG510) is a chiral compound and a potent KRAS G12C covalent inhibitor with potential antitumor activity.
The Uses of Sotorasib
Sotorasib (AMG510) is the first KRAS (G12C) inhibitor to enter clinical development. Having shown antitumor activity in clinical trials, AMG 510 represents a potentially transformative therapy.
Definition
ChEBI:Sotorasib is a pyridopyrimidine that is pyrido[2,3-d]pyrimidin-2(1H)-one substituted by 4-methyl-2-(propan-2-yl)pyridin-3-yl, (2S)-2-methyl-4-(prop-2-enoyl)piperazin-1-yl, fluoro and 2-fluoro-6-hydroxyphenyl groups at positions 1, 4, 6 and 7, respectively. It is approved for the treatment of patients with non-small cell lung cancer having KRAS(G12C) mutations. It has a role as an antineoplastic agent. It is a member of acrylamides, a N-acylpiperazine, a pyridopyrimidine, a member of monofluorobenzenes, a member of methylpyridines, a tertiary carboxamide, a tertiary amino compound and a member of phenols.
brand name
Lumakras
General Description
Sotorasib, also known as AMG-510, is an acrylamide derived KRAS inhibitor developed by Amgen. It is indicated in the treatment of adult patients with KRAS G12C mutant non small cell lung cancer. This mutation makes up >50% of all KRAS mutations. Mutant KRAS discovered in 1982 but was not considered a druggable target until the mid-2010s. It is the first experimental KRAS inhibitor. The drug [MRTX849] is also currently being developed and has the same target. Sotorasib was granted FDA approval on 28 May 2021.
General Description
Class: non-kinase; Treatment: KRAS(G12C) NSCLC; Other name: AMG 510; Elimination half-life: 5.5 h; Protein binding = 89%
Side Effects
Sotorasib may cause breathing problems that could lead to death. Get emergency medical help if you have new or worsening fever, cough, or shortness of breath.
Common side effects may include:
nausea, diarrhea;
cough;
liver problems;
pain in your bones, joints, or muscles;
tiredness;
abnormal lab tests.
For 28 patients (22%), side effects led to a pause, a reduction, or both, in the dose of sotorasib. Nine people (7%) stopped the treatment because of side effects.
www.everydayhealth.com/drugs/sotorasib
Mode of action
Sotorasib is an orally available inhibitor of the specific KRAS mutation, p.G12C, with potential antineoplastic activity. Upon oral administration, sotorasib selectively targets, binds to and inhibits the activity of the KRAS p.G12C mutant. This may inhibit growth in KRAS p.G12C-expressing tumor cells. The KRAS p.G12C mutation is seen in some tumor cell types and plays a key role in tumor cell proliferation.
Properties of Sotorasib
| Boiling point: | 730.5±70.0 °C(Predicted) |
| Density | 1.36±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C, stored under nitrogen |
| solubility | DMSO : 100 mg/mL (178.38 mM; Need ultrasonic)| |
| pka | 6.52±0.35(Predicted) |
| form | Solid |
| color | White to yellow |
| Water Solubility | Water : 33.33 mg/mL (59.46 mM; ultrasonic and adjust pH to 11 with NaOH) |
Safety information for Sotorasib
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Sotorasib
| InChIKey | NXQKSXLFSAEQCZ-UHFFFAOYSA-N |
| SMILES | O=C1N=C(N2CCN(C(=O)C=C)CC2C)C2=CC(F)=C(C3C(=CC=CC=3F)O)N=C2N1C1C(=CC=NC=1C(C)C)C |
Sotorasib manufacturer
Aspen Biopharma Labs Pvt Ltd
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2296729-00-3 Sotorasib AMG510 98%View Details
2296729-00-3 Sotorasib AMG510 98%View Details
2296729-00-3 -
 Sotorasib 2252403-56-6 / 2296729-00-3 98%View Details
Sotorasib 2252403-56-6 / 2296729-00-3 98%View Details
2252403-56-6 / 2296729-00-3 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1