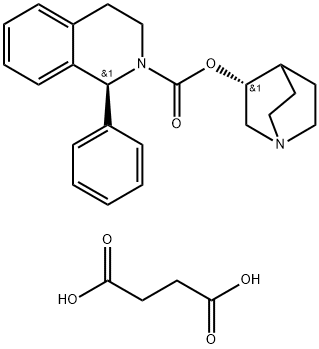
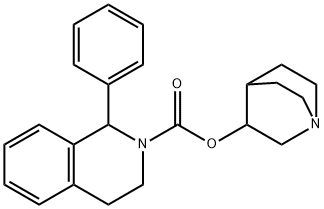
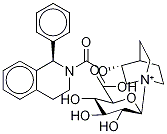
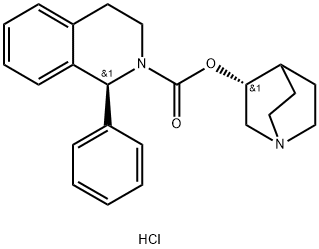
Solifenacin
- CAS NO.:242478-37-1
- Empirical Formula: C23H26N2O2
- Molecular Weight: 362.46
- MDL number: MFCD18910847
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33

What is Solifenacin?
Absorption
Solifenacin is well absorbed in the duodenum, jejunum, and ileum but not the stomach. Absorption occurs via passive diffusion and so no transporters are involved. The mean oral bioavailability of solifenacin is 88%. The Tmax of solifenacin is 3-8 hours with a Css of 32.3ng/mL for a 5mg oral dose and 62.9ng/mL for a 10mg oral dose.
Toxicity
The LD50 of Solifenacin has yet to be determined.
Signs of overdose include severe anticholinergic effects, mental status changes, and decreased consciousness. In case of overdose, treat patients with gastric lavage and supportive measures. Monitor patients with an ECG.
The Uses of Solifenacin
muscarinic M3 antagonist
Indications
Solifenacin tablets are indicated to treat an overactive bladder with urinary incontinence, urgency, and frequency.
Background
Solifenacin is a competitive muscarinic receptor antagonist indicated to treat an overactive bladder with urinary incontinence, urgency, and frequency. It has a long duration of action as it is usually taken once daily.
Solifenacin was granted FDA approval on 19 November 2004.
Definition
ChEBI: Solifenacin is a member of isoquinolines.
brand name
Vesicare (Astellas).
Pharmacokinetics
Solifenacin antagonizes the M2 and M3 muscarinic receptors in the bladder to treat an overactive bladder. It has a long duration of action as it is usually taken once daily. Patients taking solifenacin should be aware of the risks of angioedema and anaphylaxis.
Metabolism
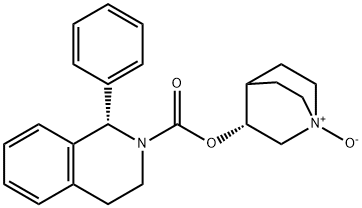
Solifenacin undergoes N-oxidation at the quinuclidin ring by cytochrome P450, though the exact enzymes are not revealed in the literature. The tetrahydroisoquinolone ring is 4R-hydroxylated by CYP3A4, CYP1A1, and CYP2D6. A 4R-hydroxy N-oxide metabolite is also formed by CYP3A4. Finally, solifenacin can undergo direct glucuronidation. Only solifenacin and the 4R-hydroxy metabolite are pharmacologically active.
Properties of Solifenacin
| Boiling point: | 505.5±50.0 °C(Predicted) |
| Density | 1.24±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | DMSO : ≥ 50 mg/mL (137.95 mM);Water : < 0.1 mg/mL (insoluble) |
| form | Solid |
| pka | 9.03±0.33(Predicted) |
| color | White to off-white |
Safety information for Solifenacin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P330:Rinse mouth. P362:Take off contaminated clothing and wash before reuse. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. P405:Store locked up. P403+P233:Store in a well-ventilated place. Keep container tightly closed. P501:Dispose of contents/container to..… |
Computed Descriptors for Solifenacin
Solifenacin manufacturer
Bondbay Pharmaceuticals Pvt Ltd
Niksan Pharmaceutical
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 242478-37-1 SolifenacinBase(1S,3’R)-3’-quinuclidinyl-1-phenyl-1,2,3,4-tetrahydro-2- isoquinolinecarboxylate(242478-37-1) 98%View Details
242478-37-1 SolifenacinBase(1S,3’R)-3’-quinuclidinyl-1-phenyl-1,2,3,4-tetrahydro-2- isoquinolinecarboxylate(242478-37-1) 98%View Details
242478-37-1 -
 Solifenacin 98%View Details
Solifenacin 98%View Details -
 Solifenacin 98%View Details
Solifenacin 98%View Details
242478-37-1 -
 Solifenacin 242478-37-1 98%View Details
Solifenacin 242478-37-1 98%View Details
242478-37-1 -
 242478-37-1 Solifenacin 98%View Details
242478-37-1 Solifenacin 98%View Details
242478-37-1 -
 242478-37-1 Solifenacin 99%View Details
242478-37-1 Solifenacin 99%View Details
242478-37-1 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8