Selenium
Synonym(s):SE000500;SE006010;SE006110;Selenium;Selenium black 99+
- CAS NO.:7782-49-2
- Empirical Formula: Se
- Molecular Weight: 78.96
- MDL number: MFCD00134090
- EINECS: 231-957-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-10 11:56:18
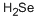
What is Selenium?
Absorption
Oral bioavailability of 90% when given as L-selenomethionine . Tmax of 9.17h.
Toxicity
Oral LD50 of 6700mg/kg in rats . Selenium exposure is teratogenic and can result in fetal death as tested in mice. Chronic toxicity is characterized by hair loss, white horizontal streaking on fingernails, paronchyia, fatigue, irritability, hyperreflexia, nausea, vomiting, garlic odor on breath, and metallic taste . Serum selenium correlates weakly with symtoms. Blood chemistry as well as liver and kidney function are normally unnaffected. Acute toxicity presents as stupor, respiratory depression, and hypotension. ST elevations and t-wave changes characteristic of myocardial infarction may be observed.
Description
Selenium was discovered in 1817 by J?ns Jacob Berzelius. Especially noted was the similarity of the new element to the previously known tellurium. Selenium is an essential trace element atw0.1 ppm in diets. Selenium is a biologically active part of a number of important proteins, particularly enzymes involved in antioxidant defense mechanisms, thyroid hormone metabolism, and redox control of intracellular reactions. In humans and animals, selenium plays a role in protecting tissues from oxidative damage as a component of glutathione peroxidase.
Chemical properties
Jewelers most frequently encounter selenium in the form of brass-black and gun-bluing compounds. Selenium print toner used by photographers is sometimes used by jewelers as a metal-coloring solution. These coloring mixtures usually contain selenic acid. Selenic acid can release hydrogen selenide gas that can cause illness, and used daily, it might enlarge the liver and spleen. Tellurium is sometimes used in association with selenium.
Chemical properties
Selenium exists in three forms: a red amor- phous powder, a gray form, and red crystals. Occurs as an impurity in most sulfide ores. Selenium, along with tellu- rium, is found in the sludges and sediments from electro- lytic copper refining. It may also be recovered in flue dust from burning pyrites in sulfuric acid manufacture.
Physical properties
Selenium is a soft metalloid or semimetal that is similar to tellurium, located just belowit in the oxygen group, and sulfur, which is just above it in the same group. Selenium hasseveral allotropic forms that range from a gray metallic appearance to a red glassy appearance.These allotropic forms also have different properties of heat, conductivity, and density. In itsamorphous state, it is a red powder that turns black and becomes crystalline when heated.Crystalline selenium has a melting point of 220°C, a boiling point of 685°C, and a densityof 4.809 g/cm3.
Isotopes
There are a total of 35 isotopes of selenium. Five of these are stable, anda sixth isotope has such a long half-life that it is also considered stable: Se-82 =0.83×10+20 years. This sixth isotope constitutes 8.73% of selenium’s abundance in theEarth’s crust, and the other five stable isotopes make up the rest of selenium’s abundanceon Earth.
Origin of Name
Named for the Greek word selene, meaning “moon.” Jons Jacob Berzelius (1779–1848) discovered selenium and named it after the mineral called “eucairite,” which in Greek means “just in time.”
Occurrence
Selenium is the 67th most abundant element in Earth’s crust. It is widely spread over theEarth, but does not exist in large quantities. As a free element it is often found with the elementsulfur.
There is only one mineral ore that contains selenium: eucairite (CuAgSe). Although rich inselenium, it is too scarce to be of commercial use. Almost all selenium is recovered from theprocessing of copper and the manufacturing of sulfuric acid as a leftover sludge by-product.This makes selenium’s recovery profitable. Recovering it from eucairite is not profitable.
Selenium is found in Mexico, Bosnia, Japan, and Canada. It can be found in recoverablequantities in some soils in many countries.
History
Discovered by Berzelius in 1817, who found it associated with tellurium, named for the Earth. Selenium is found in a few rare minerals, such as crooksite and clausthalite. In years past it has been obtained from flue dusts remaining from processing copper sulfide ores, but the anode muds from electrolytic copper refineries now provide the source of most of the world’s selenium. Selenium is recovered by roasting the muds with soda or sulfuric acid, or by smelting them with soda and niter. Selenium exists in several allotropic forms. Three are generally recognized, but as many as six have been claimed. Selenium can be prepared with either an amorphous or crystalline structure. The color of amorphous selenium is either red, in powder form, or black, in vitreous form. Crystalline monoclinic selenium is a deep red; crystalline hexagonal selenium, the most stable variety, is a metallic gray. Natural selenium contains six stable isotopes. Twentynine other isotopes and isomers have been characterized. The element is a member of the sulfur family and resembles sulfur both in its various forms and in its compounds. Selenium exhibits both photovoltaic action, where light is converted directly into electricity, and photoconductive action, where the electrical resistance decreases with increased illumination. These properties make selenium useful in the production of photocells and exposure meters for photographic use, as well as solar cells. Selenium is also able to convert a.c. electricity to d.c., and is extensively used in rectifiers. Below its melting point, selenium is a p-type semiconductor and is finding many uses in electronic and solid-state applications. It is used in xerography for reproducing and copying documents, letters, etc., but recently its use in this application has been decreasing in favor of certain organic compounds. It is used by the glass industry to decolorize glass and to make rubycolored glasses and enamels. It is also used as a photographic toner, and as an additive to stainless steel. Elemental selenium has been said to be practically nontoxic and is considered to be an essential trace element; however, hydrogen selenide and other selenium compounds are extremely toxic, and resemble arsenic in their physiological reactions. Hydrogen selenide in a concentration of 1.5 ppm is intolerable to man. Selenium occurs in some soils in amounts sufficient to produce serious effects on animals feeding on plants, such as locoweed, grown in such soils. Selenium (99.5%) is priced at about $250/kg. It is also available in high-purity form at a cost of about $350/kg (99.999%).
Characteristics
Crystalline selenium is a p-type semiconductor. It acts as a rectifier that can change electriccurrent from alternating current (AC) to direct current (to DC). It has photovoltaic proper ties, meaning it is able to convert light (radiant) energy that strikes it into electrical energy.Selenium’s resistance to the flow of electricity is influenced by the amount of light shining onit. The brighter the light, the better the electrical conductivity.
Selenium burns with a blue flame that produces selenium dioxide (SeO2). Selenium willreact with most metals as well as with nonmetals, including the elements in the halogen group17.
The Uses of Selenium
selenium is a trace mineral used for years in topical preparations for its anti-fungal properties. Selenium has been shown to have other protective effects such as repairing DnA, reducing the DnA-binding of carcinogens, and suppressing gene mutations. In laboratory studies, skin lotions containing selenium compounds have been shown to decrease uV-induced skin damage such as inflammation, blistering, and pigmentation.
The Uses of Selenium
Selenium is used in the manufacture of colored glass, in photocells, in semiconductors,as a rectifier in radio and television sets, andas a vulcanizing agent in the manufacture ofrubber.
Klaus Schwartz in 1957 discovered thattrace amounts of selenium in the feed protectedvitamin E-deficient rats from dietaryliver necrosis. Soon, thereafter, several animaland epidemiology studies showed thatits presence in the diet could provide protectiveaction in humans against several degenerativediseases including cirrhosis, cancer,diabetes and Keshan disease, a juvenile cardiomyopathy.The range between its beneficialand toxic character, however, is veryclose and, therefore, the daily dietary intakeshould be appropriately monitored (Naverro-Alarcon and Lopez-Martinez 2000). Thereis no accurate estimate of human requirementsof dietary selenium. Extrapolation ofanimal data to humans suggest an averagedaily requirement in the range 50 to 200 μg.Longnecker et al. (1991) observed no evidenceof adverse effects from selenium inhuman health at a daily intake level as highas 724 μg.
.
The Uses of Selenium
The photosensitive nature of selenium makes it useful in devices that respond to theintensity of light, such as photocells, light meters for cameras, xerography, and electric “eyes.”Selenium also has the ability to produce electricity directly from sunlight, making it ideal foruse in solar cells. Selenium possesses semiconductor properties that make it useful in the electronicsindustry, where it is a component in some types of solid-state electronics and rectifiers.It is also used in the production of ruby-red glass and enamels and as an additive to improvethe quality of steel and copper. Additionally, it is a catalyst (to speed up chemical reactions)in the manufacture of rubber.
Selenium is an essential trace element for both plants and animals, and it is a diet supplementin animal feed as well as for humans.
Background
Selenium is a trace metal in the human body particularly important as a component of glutathione peroxidase, an important enzyme in the prevention of cellular damage by free radicals and reactive oxygen species
Indications
For the supplementation of total parenteral nutrition to prevent hyposelenemia .
What are the applications of Application
Selenium is a non-metal element
Definition
A metalloid element existing in several allotropic forms and belonging to group 16 of the periodic table. It occurs in minute quantities in sulfide ores and industrial sludges. The common gray metallic allotrope is very light-sensitive and is used in photocells, solar cells, some glasses, and in xerography. The red allotrope is unstable and reverts to the gray form under normal conditions. Symbol: Se; m.p. 217°C (gray); b.p. 684.9°C (gray); r.d. 4.79 (gray); p.n. 34; r.a.m. 78.96.
Definition
selenium: Symbol Se. A metalloidelement belonging to group 16 (formerlyVIB) of the periodic table; a.n.34; r.a.m. 78.96; r.d. 4.81 (grey); m.p.217°C (grey); b.p. 684.9°C. There are anumber of allotropic forms, includinggrey, red, and black selenium. Itoccurs in sulphide ores of other metalsand is obtained as a by-product(e.g. from the anode sludge in electrolyticrefining). The element is asemiconductor; the grey allotrope islight-sensitive and is used in photocells,xerography, and similar applications.Chemically, it resemblessulphur, and forms compounds withselenium in the +2, +4, and +6 oxidation states. Selenium was discoveredin 1817 by J?ns Berzelius.
Production Methods
Selenium (Se), a nonmetallic element of the sulfur group, is
widely distributed in nature. It is obtained along with tellurium
as a by-product of metal ore refining, chiefly from
copper deposits. About 16 ton is mined a year globally.
The global refinery production of selenium, excluding the
U.S. production, increased from about 1,400 metric ton in
2000 to about 1510 metric ton in 2008 and 1500
in 2009.
Because selenium is present in fossil fuels, up to 90% of
the selenium content in ambient air is emitted during their
combustion. Air pollution concentrations averaged from
0.38 ng/m3 in remote areas to 13 ng/m3 in urban areas.
The mass medium particle diameter was 0.92 mm. The
worldwide emissions of 10,000 tons/year from natural
sources exceed the atmospheric emissions from anthropogenic
sources (5100 ton). However, 41,000 tons is emitted
into the aquatic ecosystems. The largest contributors are
electric power generating plants that produce 18,000 ton;
manufacturing processes account for 12,000 ton.
Most of the world’s selenium today is provided by
recovery from anode muds of electrolytic copper
refineries. Selenium is recovered by roasting these muds
with soda or sulfuric acid or by melting them with a soda
and niter.
General Description
Selenium is a reddish colored powder that may become black upon exposure to air. Selenium is toxic by ingestion. Selenium is used to manufacture electronic components and rubber.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
SELENIUM, silicon, or sulfur ignites in fluorine gas at ordinary temperatures [Mellor 2:11-13 1946-47]. A mixture of barium carbide and selenium heated to 150° C becomes incandescence [Mellor 5:862 1946-47]. Calcium carbide and selenium vapor react with incandescence [Mellor 5:862 1946-47]. A moist mixture of selenium and chlorates, except the alkali chlorates, becomes incandescent. Selenium reacts violently with chromium trioxide [Mellor 11:233 1946-47]. Reaction of selenium and silver bromate (also potassium bromate) is violently explosive [Mellor 2, Supp1:763 1956]. Freshly reduced selenium reacts vigorously with nitric acid. Trace amounts of organic matter probably influenced the reaction [J. Chem. Soc. 1938 p.391]. The reaction between zinc and selenium or tellurium is accompanied by incandescence [Mellor 4:476-480 1946-47].
Hazard
The fumes and gases of most selenium compounds are very toxic when inhaled. SeO2 andSeS2 are toxic if ingested and very irritating to the skin. They are also carcinogenic.
Although some compounds of selenium are poisonous, as an element it is essential in traceamounts for humans. It is recommended that 1.1 to 5 milligrams of selenium be included inthe daily diet. This amount can be maintained by eating seafood, egg yokes, chicken, milk,and whole grain cereals. Selenium assists vitamin E in preventing the breakdown of cells andsome chemicals in the human body.
Health Hazard
The toxicity of selenium and its compoundsvaries substantially. Sodium seleniteis highly toxic; many sulfur compoundsof selenium are much less toxic. The targetorgans are the respiratory tract, liver,kidneys, blood, skin, and eyes. The sign ofacute poisoning is a garlic-like odor in thebreath and sweat. The other symptoms areheadache, fever, chill, sore throat, and bronchitis.Chronic intoxication can cause loss ofhair, teeth, and nails, depression, nervousness,giddiness, GI disturbances dermititis,blurred vision, and a metallic taste. Althoughinhalation toxicity is severe in test animals,oral toxicity is of low order. Chronic exposurecould cause a disease known as selenosis,characterized by a variety of neurologicalabnormalities. The LD50 values for seleniumcompounds vary with the compounds.
Matoba et al. (1986) reported a case offatal suicidal ingestion of “Super Blue”containing 4% selenious acid. Autopsy examinationshowed highest levels of Se in thelung, kidney and stomach of the patient.Death resulted from pulmonary edema, necrosisof proximal tubules and congestion of thekidney.
Paul and coworkers (1989) have investigatedthe antidotal actions of several compoundson the acute toxicity of seleniumin rats. Male Wistar rats were injectedsodium [75Se]selenite subcutaneously inthis study. Intraperitoneal administration ofdiethyldithiocarbamate or treatment withcitrate salt of bismuth, antimony, orgermanium, administered subcutaneously,reduced selenium-induced loss of bodyweight in the animals. Germanium citrateand bis(carboxyethyl)germanium sesquioxidepromoted increases in the 24-hour urinaryexcretion of selenium when administered 15minutes after sodium selenite.
The chemical species of selenium causingits toxic actions and the molecular target,however, are not well established. Guptaand Porter (2002) attributed selenite as thepotent inhibitor of the enzyme squalenemonooxygenase in cholesterol biosynthesis.Such inhibition by selenite, as well as,methylselenol was slow and irreversibleon purified recombinant human squalenemonooxygenase thus indicating a covalentbinding to the enzyme. Their study alsoshowed that presence of dithiol enhancedthe inhibition by selenite, suggesting theformation of a more toxic species, possiblyselenide. High doses of selenite werefound to cause cytotoxicity inducing 8-hydroxydeoxy-guanosine in DNA of primaryhuman keratinocytes (Li Shen et al. 2001).These authors investigated the interaction ofselenite and selenomethionine, the commondietary selenium antioxidants (to reduceoxidative stress) with other antioxidantsin DNA damage. Synergistic effects wereobserved between selenite and trolox (awater-soluble Vitamin E). On the other handCuSo4 played a protective role in seleniteinducedcytotoxicity, DNA oxidative damage and apoptosis. Haratake et al. (2005) studiedthe reaction of glutathione selenotrisulfide,an important intermediate in the metabolismof selenite with human hemoglobin. Thestudy showed that selenotrisulfide reactedrapidly with hemoglobin under physiologicalconditions.
Fire Hazard
Combustible material: may burn but does not ignite readily. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.
Flammability and Explosibility
Non flammable
Agricultural Uses
Selenium (Se) is a metalloid element belonging to Group
16 (formerly VIB) of the Periodic Table. It is an
essential ingredient in the forage for animals to prevent
muscular dystrophy or white muscle disease which
weakens the heart of cattle and sheep.
However, selenium is not essential for plants, and its
uptake by plants varies. Certain species of Astrugals
absorb more selenium than others because of a special
amino acid in them. Plants like mustard, cabbage and
onions absorb moderate amounts of selenium. This
absorbed selenium accumulates in the tissues of these
plants, and no treatment can remove it. The excess soil
selenium content can be corrected by the addition of
barium chloride or calcium sulphate, which may form
insoluble selenate.
Chemically, selenium resembles sulphur. Its total
concentration in most soils is between 0.1 and 0.3 ppm as
selenides, elemental selenium, selenites, selenates and
organic selenium compounds. The selenium uptake is the
highest in basic soil and the lowest in neutral soil.
There has been some concern about the increased
selenium deficiencies in cattle due to a negative effect of
sulphate on the selenate ion uptake by crops. Such
livestock disorders are severe after a wet summer. This is
due to a lowered soil redox potential, converting selinium
into forms unavailable for plant uptake. This is also
pronounced in soils with increased nitrate deposition
which converts the selenate and selenite into elemental
selenium or its gaseous form. On the other hand, winter
forage is seen to contain higher amounts of selenium.
Phosphate rocks and superphosphates containing 20
ppm or more of selenium may be sufficient for plants to
protect the livestock from being deficient in selenium.
Fertilization programs to produce selenium-adequate
forage, specifically suited to grazing animals, are a
subject of continuing interest. Fertilization with selenites
is preferred to other easily available selenates in view of
the former's slow-acting nature. Fertilization with
selenites is preferred also because they produce a lesser
level of selenium in plants than selenates do. Selenium of
roughly 75 g/ha for forages and 15 g/ha for foliar
application is satisfactory.
Biotechnological Applications
Selenium is a component of a number of proteins. Selenium can exist as an anion at biological pH, which makes it able to both give and accept electrons. The best understood physiological functions of selenium are two enzyme functions. One of these functions is done as part of a family of proteins named glutathione peroxidase (one is found inside of cells, another is outside cells in places like the plasma).
Glutathione peroxidase is part of the body's antioxidant defense network by eliminating peroxides, including hydrogen peroxide, which can be both precursors and products of free radicals. Selenium also functions in an enzyme that is part thyroid hormone synthesis. A more recently discovered selenium enzyme is known as thioredoxin reductase, which seems to have a number of regulatory roles within cells, and seems to affect antioxidant defense by inßuencing electron ßow in some reactions. One interesting point about this enzyme is that in rats, the enzyme activities can be increased by elevating selenium intake above those normally considered adequate.
Pharmacokinetics
Selenium is incorporated into many different selenoproteins which serve various functions throughout the body .
Safety Profile
Poison by intravenous route. When heated to decomposition it emits toxic fumes of Se. See also SELENIUM and SELENIUM COMPOUNDS
Potential Exposure
Most of the selenium produced is used in the manufacture of selenium rectifiers. It is also utilized as a pigment for ruby glass, paints, and dyes; as a vulcaniz- ing agent for rubber; a decolorizing agent for green glass; a chemical catalyst in the Kjeldahl test; as an insecticide; in the manufacture of electrodes, selenium photocells, sele- nium cells, and semiconductor fusion mixtures; in photo- graphic toning bathes; and for dehydrogenation of organic compounds. It is also used in veterinary medicine and in antidandruff shampoos. Se is used in radioactive scanning for the pancreas and for photostatic and X-ray xerography. It may be alloyed with stainless steel; copper, and cast steel. Selenium is a contaminant in most sulfide ores of copper, gold, nickel, and silver; and exposure may occur while removing selenium from these ores.
Veterinary Drugs and Treatments
Depending on the actual product and species, vitamin E/selenium
is indicated for the treatment or prophylaxis of selenium-tocopherol
deficiency (STD) syndromes in ewes and lambs (white muscle
disease), sows, weanling and baby pigs (hepatic necrosis, mulberry
heart disease, white muscle disease), calves and breeding cows
(white muscle disease), and horses (myositis associated with STD).
Vitamin E may be useful as adjunctive treatment of discoid lupus
erythematosus, canine demodicosis, and acanthosis nigricans in dogs. It may also be of benefit in the adjunctive treatment of hepatic
fibrosis or adjunctive therapy of copper-associated hepatopathy
in dogs.
Environmental Fate
Although selenium occurs naturally in the environment found
in rocks and soil, it can also be released by both natural and
manufacturing processes. However, forms of selenium can be
transformed (changed) in the environment. Weathering of
rocks to soil may cause low levels of selenium in water or it may
cause it to be taken up by plants and naturally released into the
air. Volcanic eruptions are suspected of contributing to selenium
in air, and soils in the areas around volcanoes tend to
have enriched amounts of selenium.
Selenium has multiple oxidation states (valence states)
including -2, 0, +4, and +6. The type of selenium found is
a result of its oxidation state, which may vary according to
ambient conditions, such as pH and microbial activity. Selenium
enters the air from burning coal or oil. Most of the selenium in
air is bound to fly ash and to suspended particles. The elemental
selenium that may be present in fossil fuels forms selenium
dioxide during combustion (burning). Selenium dioxide can
then form selenious acid with water or sweat. Selenium anhydride is released during the heating of copper, lead, and zinc
ores when there is selenium in them. Hydrogen selenide
decomposes rapidly in air to form elemental selenium and
water, thus eliminating the danger from this compound formost
people, except those who are exposed to it in their workplace.
Airborne particles of selenium, such as in coal ash, can settle
on soil or surface water. Disposal of selenium in commercial
products and waste could also contribute to selenium levels in
soil. But the amount of selenium released to soil from fly ash
and hazardous waste sites has not been measured. The forms
and fate of selenium in soil depend largely on the acidity of the
surroundings and its interaction with oxygen. In theory,
at equilibrium with no oxygen present, deep-soil selenium may
be present as elemental selenium. In the absence of oxygen
when the soil is acidic, the amount of biologically available
selenium should be low. Elemental selenium that cannot
dissolve in water and other insoluble forms of selenium (such
as selenium sulfide and heavy metal selenides) are less mobile
and will usually remain in the soil, posing less of a risk for
exposure. Active agricultural or industrial processes may
increase the amount of biologically available selenium by
decreasing the acidity of the soil and increasing the oxygen and
the soluble selenium compounds. Selenium compounds that
can dissolve in water are very mobile. For example, selenates
and selenites are water-soluble, and thus mobile, so there is an
increased chance of exposure to them. Irrigation drainage
waters may result in increased selenium entering the surface
water. Other factors that may affect the rates at which selenium
moves through the soil are temperature, moisture, time, season
of year, concentration of water-soluble selenium, organic
matter content, and microbiological activity.
Metabolism
Selenium supplements are typically available in the form of sodium selenite which is metabolized to selenide through either glutathione conjugation and subsequent reduction by glutathione reductase enzymes or reduction by thioredoxin reductases . Selenide is further metabolized to selenocystein by cysteine synthases and to selenophosphate by selenophosphate synthases. Selenide is also metabolized progressively to methyl-selenol, dimethyl selenide, then trimethylselenonium. Selenocysteine is degraded to methyl-selenol, pyruvate and ammonia by cysteine beta lyase. Selenocystein reacts with oxygen to form selenocysteine selenoxide which spontaneously degrades to methylselenic acid, pyruvate and ammonia. Methylselenic acid can be converted to methylselenol via conjugation with thiol groups on proteins like glutathione.
Shipping
UN3283 Selenium compound, solid, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous material, Technical Name Required.
Purification Methods
Dissolve selenium in small portions in hot conc HNO3 (2mL/g), filter and evaporate to dryness to give selenious acid which is then dissolved in conc HCl. Pass SO2 gas through the solution whereby selenium (but not tellurium) precipitates. It is filtered off and washed with conc HCl. This purification process is repeated. The selenium is then converted twice to the selenocyanate by treating with a 10% excess of 3M aqueous KCN (CARE), heated for half an hour on a sand-bath and filtered. Add an equal weight of crushed ice to the cold solution, followed by an excess of cold, conc HCl, with stirring (in an efficient fume cupboard as HCN is evolved) which precipitates selenium powder. This is washed with water until colourless, and then with MeOH and is heated in an oven at 105o. Finally it is fused for 2hours in vacuo. It is cooled, crushed and stored in a desiccator [Tideswell & McCullough J Am Chem Soc 78 3036 1956].
Toxicity evaluation
Selenium in the body can be grouped in three main categories:
selenium in proteins, nonprotein selenium species, and selenoamino
acids. The most prevalent selenium species include
selenocysteine, selenomethionine, and inorganic forms of selenium
(selenite and selenate). Little is known about the specific
biochemical mechanisms by which selenium and selenium
compounds exert their acute toxic effects but may involve redox
cycling. Generally, water-soluble forms are more easily absorbed
and are generally of greater acute toxicity. Sulfhydryl enzymes are
attacked by soluble selenium compounds.
Excess selenium results in liver atrophy, necrosis, and
hemorrhage.
Structure and conformation
It takes three types of structures, metallic (grey) selenium, crystal (red) selenium, and amorphous selenium. The space lattice of metallic selenium belongs to the hexagonal system with two types, A and B. The B type is the most stable and the quasi-stable; A type changes to the B type slowly. The structure of the B type is an infinite zigzag chain containing three atoms in a unit cell with lattice constant of a=0.4355 nm, c=0.4949 nm, Se–Se=0.232 nm, and <Se– Se–Se=105° . The space lattice of crystal belongs to the monoclinic system with a=0.905 nm, b=0.907 nm, c=1.161 nm, Se–Se=0.234 nm, b=90841' , and <Se–Se–Se=105.38° ±2.3° . There may be two types for crystal selenium. Amorphous selenium changes to metallic selenium slowly at room temperature. Sometimes, selenium is classified into trigonal and amorphous types3 .
Incompatibilities
Reacts violently with strong acids and strong oxidizers, chromium trioxide; potassium bromate;cadmium. Reacts with incandescence on gentle heating with phosphorous and metals, such as nickel, zinc, sodium, potassium, platinum. Reacts with water @ 50 ? C forming flammable hydrogen and selenious acids.
Waste Disposal
Powdered selenium: dispose in a chemical waste landfill. When possible, recover selenium and return to suppliers
Precautions
During use and handling of selenium, occupational workers should be careful to avoid contact with the skin. Selenium compounds are considered very damaging to the liver, and hazardous.
Properties of Selenium
| Melting point: | 217 °C (lit.) |
| Boiling point: | 684.9 °C (lit.) |
| Density | 4.81 g/mL at 25 °C (lit.) |
| vapor pressure | <1 Pa (20 °C) |
| storage temp. | no restrictions. |
| solubility | H2O: soluble |
| form | powder |
| Specific Gravity | 4.81 |
| color | White to creamy white |
| Resistivity | 1.2 μΩ-cm, 0°C |
| Water Solubility | Insoluble |
| Merck | 13,8505 |
| Exposure limits | TLV-TWA 0.2 mg(Se)/m3 (ACGIH, MSHA,
and OSHA); IDLH 100 mg/m3. |
| Dielectric constant | 6.1(Ambient) |
| Stability: | Stable. Incompatible with strong acids, strong oxidizing agents and most common metals. Combustible. |
| CAS DataBase Reference | 7782-49-2(CAS DataBase Reference) |
| IARC | 3 (Vol. 9, Sup 7) 1987 |
| NIST Chemistry Reference | Selenium atom(7782-49-2) |
| EPA Substance Registry System | Selenium (7782-49-2) |
Safety information for Selenium
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H373:Specific target organ toxicity, repeated exposure H413:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P314:Get medical advice/attention if you feel unwell. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Selenium
| InChIKey | SPVXKVOXSXTJOY-UHFFFAOYSA-N |
Selenium manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
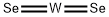
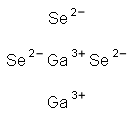
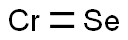
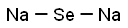
You may like
-
 Selenium CAS 7782-49-2View Details
Selenium CAS 7782-49-2View Details
7782-49-2 -
 Selenium CAS 7782-49-2View Details
Selenium CAS 7782-49-2View Details
7782-49-2 -
 Selenium CAS 7782-49-2View Details
Selenium CAS 7782-49-2View Details
7782-49-2 -
 Selenium CAS 7782-49-2View Details
Selenium CAS 7782-49-2View Details
7782-49-2 -
 Selenium CAS 7782-49-2View Details
Selenium CAS 7782-49-2View Details
7782-49-2 -
 Selenium CAS 7782-49-2View Details
Selenium CAS 7782-49-2View Details
7782-49-2 -
 Selenium CAS 7782-49-2View Details
Selenium CAS 7782-49-2View Details
7782-49-2 -
 Selenium Pharmaceutical ChemicalsView Details
Selenium Pharmaceutical ChemicalsView Details
7782-49-2
