Sodium pyruvate
Synonym(s):Pyruvic acid sodium;Pyruvic acid sodium salt;Sodium pyruvate, Pyruvate;Α-ketopropionic acid sodium salt;α-Ketopropionic acid sodium salt
- CAS NO.:113-24-6
- Empirical Formula: C3H5NaO3
- Molecular Weight: 112.06
- MDL number: MFCD00002586
- EINECS: 204-024-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
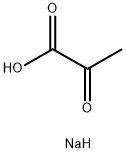
What is Sodium pyruvate?
Description
Sodium pyruvate is the sodium salt of pyruvate. It is frequently supplemented to the cell culture medium to act as a source of energy since it is a key intermediate during the production of the high-energy ATP molecules inside cells. For example, it can be used as a carbon source for bacteria. It may also protect cell against hydrogen peroxide, an oxidant to scavenger oxygen radicals. It is an important metabolic intermediate in many essential metabolic pathways such as carbohydrate metabolism. For example, it is converted into acetyl coenzyme A and enters into the TCA cycle (Kreb’s cycle) in organisms. It is also involved in the amino acid metabolism in organisms.
Chemical properties
White to slightly yellow crystalline powder. soluble in water, very slightly soluble in ethanol.
The Uses of Sodium pyruvate
Sodium pyruvate is used in cell culture media as an additional source of energy. It acts as an antioxidant and finds protective effects against oxygen radicals. It serves as an intermediate in many metabolic pathways such as sugar metabolism. It is converted into acetyl coenzyme A and enters the trichloroacetic acid cycle. In addition, it is involved with amino acid metabolism and initiates the Kreb's cycle. It plays an important role as a free radical scavenger.
The Uses of Sodium pyruvate
Intermediate in sugar metabolism and in enzymatic carbohydrate degradation (alcoholic fermentation) where it is converted to acetaldehyde and CO2 by carboxylase. In muscle, Pyruvic acid (derived from glycogen) is reduced to lactic acid during exertion, which is reoxidized and partially retransformed to glycogen during rest. The liver can convert Pyruvic acid to alanine by amination. A diagnostic agent for Parkinson disease
What are the applications of Application
Sodium pyruvate is an intermediate of sugar metabolism, an antioxidant, and an apoptosis inhibitor
What are the applications of Application
Sodium Pyruvate Solution 100 mM (100X) is a component in culture broth and media
What are the applications of Application
Sodium pyruvate has been used:
To prepare Bull Tyrode′s albumin-lactate-pyruvate diluent.
In pyruvate tolerance test.
To prepare oligodendrocyte culture medium.
Preparation
The preparation of sodium pyruvate is as follows:Synthesis of pyruvate, a salt of a compound of formula (II) by ozonolysis of methacrylic acid, a compound of formula (II) A solution of methacrylic acid obtained in Example la or lb (15.31 g, 178 mmol) in dichloromethane/methanol (5 % methanol, 50 ml) was cooled to -78 0C and a stream of ozone was passed through until the solution turned blue. Dimethylsulfide (12.41 g, 200 mmol) was added and the mixture was allowed to reach room temperature. Excess dimethylsulfide was removed by passing a stream of nitrogen through the reaction mixture. The reaction mixture was evaporated in vacuo at 30 0C. To the residue was added water (100 ml) and then slowly added a solution of aq. NaOH (IM, 178 ml). The mixture was concentrated and left for crystallisation at 4 °C. The white crystalline material obtained was filtered and washed with acetone (3x100 ml). Yield: 14.50 g (92 %). Purity: 95 %.
Definition
ChEBI: Sodium pyruvate is an organic sodium salt. It contains a pyruvate.
Biological Functions
Sodium pyruvate (α-Ketopropionic acid sodium salt; 2-Oxopropanoic acid sodium salt;Pyruvic acid sodium; C3H3NaO3) as an important endogenous small molecules participates various tissue and organ metabolism processes that is the final product of glycolysis and the starting substrate for the tricarboxylic acid (TCA) cycle, and possesses antioxidant and scavenging free radical effects, thus widely using as buffer, excipient and antioxidant in medicine, diagnostic reagent and medical device.
Sodium pyruvate is an endogenous antioxidant and reactive oxygen radical scavenger. In this process, H2O2 or other reactive oxygen radicals are scavenged by sodium pyruvate by a nonenzymatic reaction or an oxidative dephosphorylation, and produce acetate, water and carbon dioxide. Thus, sodium pyruvate can suppress renal cellular injury induced by H2O2, such as lipid peroxidation of rat kidney homogenate, and cytosolic 51Cr release (a marker of cellular injury) from renal epithelial cells induced by H2O2. Thus, sodium pyruvate as effect antioxidant has potential in the clinical medication. Moreover, as effect antioxidant, sodium pyruvate is also as additives used in a vast range of foods and toiletries.
General Description
Sodium pyruvate (SP) is a simplest keto-acid. It crystallizes as very thin plates and belongs to the monoclinic system. Its utility in Knoevenagel condensation carried out in an aqueous medium has been examined. The standard molar enthalpy of formation and molar enthalpy of dissolution of SP at infinite dilution have been obtained. Its efficacy in treating mitochondrial DNA depletion syndromes has been investigated.
Flammability and Explosibility
Not classified
Biochem/physiol Actions
Sodium pyruvate is known to be an effective therapeutic target for type II citrullinaemia which is characterized by hyperammonaemia. In vivo studies proves that sodium pyruvate has protective function against hemorrhagic shock by preventing lipid peroxidation, NAD+ reduction and cleavage of poly(ADP-ribose) polymerases. Sodium pyruvate is also known to possess anti-inflammatory action and might show improvement in chronic lung disorder.
storage
Sterile filtered commercial solutions of sodium pyruvate are stable up to 24 months, when stored at 2-8 °C.
Pyruvic acid polymerizes and decomposes upon standing. It is advised to keep containers tightly sealed.
References
https://en.wikipedia.org/wiki/Sodium_pyruvate
Taidi, Behnam, et al. "Effect of carbon source and concentration on the molecular mass of poly (3-hydroxybutyrate) produced by Methylobacterium extorquens and Alcaligenes eutrophus." Applied microbiology and biotechnology 40.6 (1994): 786-790.
https://www.alfa.com/zh-cn/catalog/A11148/
http://bio.lonza.com/uploads/tx_mwaxmarketingmaterial/Lonza_BenchGuides_Sodium_Pyruvate_Solution_100mM.pdf
Properties of Sodium pyruvate
| Melting point: | >300 °C (lit.) |
| Boiling point: | >300℃ |
| Density | 1,267g/cm |
| vapor pressure | 0Pa at 20℃ |
| refractive index | 1,426-1,43 |
| storage temp. | 2-8°C |
| solubility | H2O: 100 mg/mL |
| form | solution |
| color | White to Pale Yellow |
| PH | pH(100g/l, 25℃) : 4.0~8.0 |
| Water Solubility | Soluble in water. |
| BRN | 3568341 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 113-24-6(CAS DataBase Reference) |
| EPA Substance Registry System | Sodium pyruvate (113-24-6) |
Safety information for Sodium pyruvate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Sodium pyruvate
| InChIKey | DAEPDZWVDSPTHF-UHFFFAOYSA-M |
Sodium pyruvate manufacturer
JSK Chemicals
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



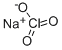
You may like
-
 Sodium pyruvate 99%View Details
Sodium pyruvate 99%View Details -
 Sodium pyruvate 95% CAS 113-24-6View Details
Sodium pyruvate 95% CAS 113-24-6View Details
113-24-6 -
 Sodium pyruvate CAS 113-24-6View Details
Sodium pyruvate CAS 113-24-6View Details
113-24-6 -
 Sodium pyruvate CAS 113-24-6View Details
Sodium pyruvate CAS 113-24-6View Details
113-24-6 -
 Sodium pyruvate, suitable for cell culture CAS 113-24-6View Details
Sodium pyruvate, suitable for cell culture CAS 113-24-6View Details
113-24-6 -
 Sodium Pyruvate CASView Details
Sodium Pyruvate CASView Details -
 Sodium Pyruvate CAS 113-24-6View Details
Sodium Pyruvate CAS 113-24-6View Details
113-24-6 -
 Sodium Pyruvate CAS 113-24-6View Details
Sodium Pyruvate CAS 113-24-6View Details
113-24-6