Sodium lauryl ether sulfate
- CAS NO.:68585-34-2
- Empirical Formula: C12H26Na2O5S
- Molecular Weight: 328.38
- MDL number: MFCD01772167
- EINECS: 500-223-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:17

What is Sodium lauryl ether sulfate?
The Uses of Sodium lauryl ether sulfate
Sodium Lauryl Ether Sulfate can be used for wood coating formulation.
The Uses of Sodium lauryl ether sulfate
Sodium laureth sulfate (sometimes referred to as SLES) is used in cosmetics as a detergent and also to make products bubble and foam. It is common in shampoos, shower gels and facial cleansers. It is also found in household cleaning products, like dish soap.
Definition
Sodium lauryl ether sulfate(SLES) is an anionic surfactant which is widely used in rinse off products as a primary surfactant. In addition to excellent detergency (also referred as cleansing), it also has excellent emulsification and foamability. It is major component of rinse-off products. It is compatible with all surfactants except cationic.
Benefits
The product has good solvency, favorable hard-water resistance and high-biodegradation. It also facilitates ease of formulation and production. In addition, it also creates a degree of thickening to the final product formulation.
Hazard
sodium laureth sulfate may be contaminated with measurable amounts of ethylene oxide and 1,4-dioxane. The International Agency for Research on Cancer ethylene oxide as a known human carcinogen and 1,4-dioxane as a possible human carcinogen. Ethylene oxide can also harm the nervous system and the California Environmental Protection Agency has classified it as a possible developmental toxicant based on evidence that it may interfere with human development. 1,4-dioxane is also persistent. In other words, it doesn’t easily degrade and can remain in the environment long after it is rinsed down the shower drain. 1,4-dioxane can be removed from cosmetics during the manufacturing process by vacuum stripping, but there is no easy way for consumers to know whether products containing sodium laureth sulfate have undergone this process. The industry panel that reviews the safety of cosmetics ingredients notes that sodium laureth sulfate can irritate the skin and eyes (though approving of its use in cosmetics).
Side Effects
Sodium lauryl ether sulfate(SLES) can irritate eyes, skin, and lungs, especially with long-term use. It may also be contaminated with a substance called 1,4-dioxane, which is known to cause cancer in laboratory animals. This contamination occurs during the manufacturing process.
Synthesis
Sodium lauryl ether sulfate is prepared by ethoxylation of dodecyl alcohol, which is produced industrially from palm kernel oil or coconut oil. The resulting ethoxylate is converted to a half ester of sulfuric acid, which is neutralized by conversion to the sodium salt. The related surfactant sodium lauryl sulfate or SLS (also known as sodium dodecyl sulfate or SDS) is produced similarly, but without the ethoxylation step.
Properties of Sodium lauryl ether sulfate
| storage temp. | Hygroscopic, Refrigerator, Under inert atmosphere |
| solubility | Chloroform (Slightly), Methanol (Sparingly), Water (Slightly) |
| form | Gel |
| color | Colourless to Off-White |
| Stability: | Hygroscopic |
| InChI | InChI=1S/C12H26O.2Na.H2O4S/c1-2-3-4-5-6-7-8-9-10-11-12-13;;;1-5(2,3)4/h13H,2-12H2,1H3;;;(H2,1,2,3,4)/q;2*+1;/p-2 |
| CAS DataBase Reference | 68585-34-2(CAS DataBase Reference) |
| EPA Substance Registry System | Polyethylene glycol mono-C10-16-alkyl ether sulfate sodium salt (68585-34-2) |
Safety information for Sodium lauryl ether sulfate
Computed Descriptors for Sodium lauryl ether sulfate
| InChIKey | SEKVTWKYOIVNGT-UHFFFAOYSA-L |
| SMILES | [Na+].[Na+].S([O-])([O-])(=O)=O.OCCCCCCCCCCCC |
Sodium lauryl ether sulfate manufacturer
Bazayan & Co.
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
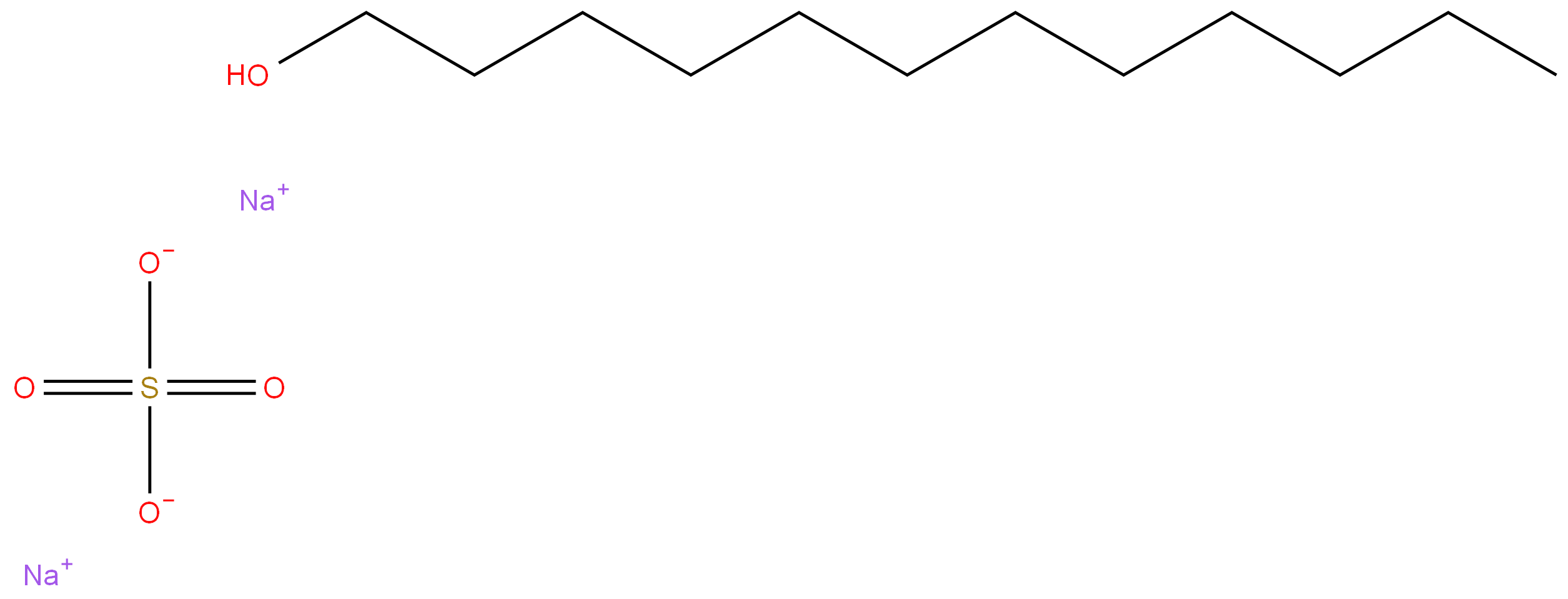 68585-34-2 SODIUM LAURYL ETHER SULPHATE 99%View Details
68585-34-2 SODIUM LAURYL ETHER SULPHATE 99%View Details
68585-34-2 -
 Sodium Lauryl Ether Sulfate 99%View Details
Sodium Lauryl Ether Sulfate 99%View Details -
 Sodium Lauryl Ether Sulfate 98%View Details
Sodium Lauryl Ether Sulfate 98%View Details -
 Sodium Lauryl Ether Sulfate 98%View Details
Sodium Lauryl Ether Sulfate 98%View Details -
 SLES 70%View Details
SLES 70%View Details
68585-34-2 -
 Sodium Lauryl Ether Sulfate Sles 70, Paste 70%View Details
Sodium Lauryl Ether Sulfate Sles 70, Paste 70%View Details
68891-38-3 -
 Sodium Lauryl Ether Sulfate, Paste 70%View Details
Sodium Lauryl Ether Sulfate, Paste 70%View Details
68585-34-2 -
 Sodium Lauryl Ether SulfateView Details
Sodium Lauryl Ether SulfateView Details
68585-34-2
