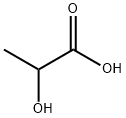
Sodium lactate
Synonym(s):(±)-2-Hydroxypropionic acid sodium salt;DL -Lactic acid sodium salt
- CAS NO.:72-17-3
- Empirical Formula: C3H5NaO3
- Molecular Weight: 112.06
- MDL number: MFCD00065400
- EINECS: 200-772-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:55
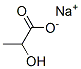
What is Sodium lactate?
Description
Sodium lactate is the sodium salt of lactic acid that has a mild saline taste. It is produced by fermentation of a sugar source, such as corn or beets, and then, by neutralizing the resulting lactic acid to create a compound having the formula NaC3H5O3
As early as 1836, sodium lactate was recognized as a salt of a weak acid rather than being a base, and it was then known that the lactate had to be metabolized in the liver before the sodium could have any titrating activity.
Chemical properties
colourless liquid
Chemical properties
Sodium lactate occurs as a clear, colorless, slightly syrupy liquid. It is odorless, or has a slight odor with a characteristic saline taste. It is hygroscopic.
The Uses of Sodium lactate
Instead of glycerol in calico printing; as a plasticizer for casein; as a corrosion inhibitor in alcohol antifreeze mixture.
The Uses of Sodium lactate
The sodium salt of lactic acid (L113490). It acts as a preservative, acidity regulator, and bulking agent.
What are the applications of Application
Sodium DL-lactate solution is for inclusion in standard lactated Ringer′s solution. It has been used as a component in Trypticase soy broth to culture P. gingivalis.
What are the applications of Application
Sodium DL-lactate is a biochemical with approximately equal amounts of D and L isomers
Definition
ChEBI: An organic sodium salt having lactate as the counterion.
General Description
Sodium DL-lactate is an organic, sodium salt of lactic acid. It is a hygroscopic agent and a component of natural moisturizing factor (NMF). It is usually produced by fermentation.
Pharmaceutical Applications
Sodium lactate is widely used in cosmetics, food products and
pharmaceutical applications including parenteral and topical
formulations.
Therapeutically, sodium lactate is used in infusions as a
component of Ringer-lactate solution; as an alternative for sodium
hydrogencarbonate in light acidosis; as a rehydrating agent; and as
a carrier for electrolyte concentrates or medicines in perfusion/
infusion solutions.
Biochem/physiol Actions
Sodium lactate functions as a buffer to maintain osmotic and charge balance. It serves as a humectant, especially in cosmetic preparations. It acts a flavoring agent in meat and poultry products. Sodium lactate also plays a role as a buffer. Sodium lactate could be an alternative source of energy and an oxidative substrate in case of lack of oxygen in brain cells.
The Uses of Sodium lactate
As a food additive, sodium lactate has the E number E325 and is naturally a liquid product, but also is available in powder form. It acts as a preservative, acidity regulator, and bulking agent.
Sodium lactate is sometimes used in shampoo products and other similar items such as liquid soaps as it is an effective humectant and moisturizer.
Sodium lactate is used to treat arrhythmias caused by overdosing of class I antiarrythmics, as well as pressor sympathomimetics which can cause hypertension.
It also can be given intravenously as a source of bicarbonate for preventing or controlling mild to moderate metabolic acidosis in patients with restricted oral intake (for sodium bicarbonate) whose oxidative processes are not seriously impaired. However, the use in lactic acidosis is contraindicated.
Food additive
Sodium lactate need not be restricted by someone avoiding milk or those with a milk allergy.In general, lactates such as sodium, calcium, and potassium lactate are salts derived from the neutralization of lactic acid and most commercially used lactic acids are fermented from dairy - free products such as cornstarch, potatoes, or molasses. Sugar or tapioca additionally may be used. However some lactic acid is fermented from dairy products such as whey and lactose . Whey is made of up 6.5 % solids of which 4.8% is solid lactose . Waste whey typically is used to produce lactic acid when the whey itself is produced as waste during the manufacture of certain dairy products . As a result, such dairy-type lactic acid generally goes back into dairy products, such as ice cream and cream cheese, rather than into non - dairy products. Moreover, although the lacticacid starter culture to ferment corn or beets may contain milk, sodium lactate does not contain milk protein and need not be restricted by someone avoiding milk or those with a milk allergy.
Safety
Sodium lactate occurs naturally in the body and is involved in
physiological processes. It is generally regarded as a relatively
nontoxic and nonirritant material when used as an excipient. Low
concentrations are well tolerated by skin and eye mucosa, although
higher concentrations should be avoided.
LD50 (rat, IP): 2 g/kg
Storage
Sodium lactate should be stored in a well-closed container in a cool, dry, place. Sodium lactate is combustible and decomposes upon heating.
Regulatory Status
GRAS listed (not for infant formulas). Included in the FDA Inactive Ingredient Database (epidural, IM, IV, and SC injections; oral suspensions; topical gels and solutions). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Properties of Sodium lactate
| Melting point: | 17°C |
| Boiling point: | 110°C |
| Density | 1.33 |
| vapor density | 0.7 (vs air) |
| vapor pressure | 17.535 mm of Hg (@ 20°C) |
| refractive index | 1.422-1.425 |
| storage temp. | 2-8°C |
| solubility | Miscible with ethanol (95%), and with water. |
| form | syrup |
| color | Light Yellow |
| Odor | Odorless |
| PH | pH (7→35, 25℃) : 6.5~7.5 |
| PH Range | 6.5 - 8.5 |
| Water Solubility | miscible |
| Merck | 14,8635 |
| BRN | 4332999 |
| Stability: | Stable. |
| CAS DataBase Reference | 72-17-3(CAS DataBase Reference) |
| EPA Substance Registry System | Sodium lactate (72-17-3) |
Safety information for Sodium lactate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Sodium lactate
Sodium lactate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Sodium lactate 72-17-3 98%View Details
Sodium lactate 72-17-3 98%View Details
72-17-3 -
 Sodium DL-Lactate (60% in Water) CAS 72-17-3View Details
Sodium DL-Lactate (60% in Water) CAS 72-17-3View Details
72-17-3 -
 Sodium Lactate, Syrup, 60% (w/w) CASView Details
Sodium Lactate, Syrup, 60% (w/w) CASView Details -
 Sodium lactate 60% solution CAS 72-17-3View Details
Sodium lactate 60% solution CAS 72-17-3View Details
72-17-3 -
 Sodium lactate 60% solution CAS 72-17-3View Details
Sodium lactate 60% solution CAS 72-17-3View Details
72-17-3 -
 SODIUM LACTATE 60% SOLUTION Extra Pure CAS 72-17-3View Details
SODIUM LACTATE 60% SOLUTION Extra Pure CAS 72-17-3View Details
72-17-3 -
 Sodium DL-lactate solution CAS 72-17-3View Details
Sodium DL-lactate solution CAS 72-17-3View Details
72-17-3 -
 Sodium DL-lactate CAS 72-17-3View Details
Sodium DL-lactate CAS 72-17-3View Details
72-17-3
