Sodium fusidate
Synonym(s):Fucidin;Fusidic acid sodium salt
- CAS NO.:751-94-0
- Empirical Formula: C31H49NaO6
- Molecular Weight: 540.72
- MDL number: MFCD00865135
- EINECS: 212-030-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
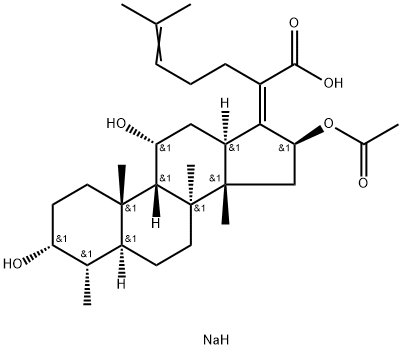
What is Sodium fusidate?
Description
Fusidic acid is a fusidane antibiotic originally isolated from F. coccineum. It is active against the Gram-positive bacteria S. aureus, S. pyogenes, C. diphtheriae, B. subtilis, and C. tetani (MIC50s = 0.01-20 μg/ml) but not the Gram-negative bacteria E. coli, S. typhimurium, and P. vulgaris or the fungi C. albicans and A. fumigatus (MIC50s = >100 μg/ml for all). Fusidic acid inhibits ribosomal recycling and protein translocation, processes mediated by elongation factor G (EF-G), in isolated E. coli ribosomes (IC50s = ~0.1 and ~200 μM, respectively). Topical administration of fusidic acid (2%) reduces the number of skin colony forming units (CFUs) and levels of TNF-α and IL-6 in a mouse model of methicillin-resistant S. aureus (MRSA) skin wound infection.
Chemical properties
White or almost white, crystalline powder, slightly hygroscopic.
The Uses of Sodium fusidate
Sodium fusidate is the more water soluble sodium salt of fusidic acid, a steriodal metabolite of Fusidum coccineum which is a potent Gram positive antibiotic. Fusidic acid inhibits protein synthesis in prokaryotes by inhibiting the ribosome-dependent activity of G factor and translocation of peptidyl-tRNA. Fusidic acid also suppresses NO lysis of pancreatic islet cells.
The Uses of Sodium fusidate
Antibacterial;Protein synthesis inhibitor GTPase coupled
The Uses of Sodium fusidate
Sodium fusidate is the more water soluble sodium salt of fusidic acid, a steroidal metabolite of Fusidum coccineum which is a potent Gram positive antibiotic. Fusidic acid inhibits protein synthesis in prokaryotes by inhibiting the ribosome-dependent activity of G factor and translocation of peptidyl-tRNA. Fusidic acid also suppresses NO lysis of pancreatic islet cells.
What are the applications of Application
Fusidic acid sodium salt is an antibiotic, nitric oxide suppressor, and protein synthesis inhibitor
brand name
Fucidine (Bristol-Myers Squibb).
General Description
Fusidic acid is an antibiotic substance, resembling the structure of a steroid. It belongs to the class of fusidanes.
Biochem/physiol Actions
Fusidic acid possesses antimicrobial action against skin pathogens. It is highly specific to Staphylococcus aureus. It is therefore used for treating infected traumatic wounds, folliculitis, furunculosis, impetigo, erythrasma and abscesses.
Clinical Use
Antibacterial agent
in vitro
resistance to fusidic acid in s. aureus strains was selected and mutations were identified in the fusa gene, which encodes ef-g. fusidic acid could not bind to free ef-g, but rather to ef-g gtp in the complex with ribosome, suggesting that the antibiotic required a specific conformation of ef-g for binding. biochemically, fusidic acid permited ribosome-stimulated gtp hydrolysis by ef-g, but prevented the associated conformational changes in ef-g, and therefore prevented ef-g turnover by stabilizing ef-g gdp on the ribosome. fusidic acid did not work on eukaryotes, but sordarin was considered to similarly act on yeast ef2 and was used to assemble ef2-80s yeast ribosome complexes for cryo-em analysis [1].
in vivo
animal study found that fusidic acid sodium salt was well absorbed after oral administration and it could significantly reduce the diabetes incidence in bb rats. fusidic acid sodium salt substantially accumulated more in female rats which might refult from the steroid structure of fusidin [2].
Drug interactions
Potentially hazardous interactions with other drugs
Antivirals: concentration of both drugs increased in
combination with ritonavir and saquinavir - avoid
with ritonavir.
Statins: increased risk of myopathy with simvastatin
and atorvastatin especially in diabetics. Avoid with
simvastatin and for 7 days after last dose.1
Metabolism
Fusidic acid is excreted in the bile, almost entirely as
metabolites, some of which have weak antimicrobial
activity.
Approximately 2% appears unchanged in the faeces.
References
[1] wilson, d. n. the a-z of bacterial translation inhibitors. crit. rev. biochem. mol. biol. 44(6), 393-433 (2009).
[2] hageman i, buschard k. antidiabetogenic effect of fusidic acid in diabetes prone bb rats: a sex-dependent organ accumulation of the drug is seen. pharmacol toxicol. 2002 sep;91(3):123-8.
Properties of Sodium fusidate
| Melting point: | >200°C (dec.) |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | H2O: soluble |
| form | neat |
| form | Solid |
| color | White to Off-White |
| Water Solubility | Soluble in water |
| Sensitive | Moisture & Light Sensitive |
| Merck | 13,4339 |
| CAS DataBase Reference | 751-94-0(CAS DataBase Reference) |
Safety information for Sodium fusidate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for Sodium fusidate
Sodium fusidate manufacturer
Allmpus Laboratories Pvt Ltd
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran

You may like
-
 FUSIDIC ACID SODIUM SALT / SODIUM FUSIDATE / FUSIDIC ACID SODIUM SALT 95.65View Details
FUSIDIC ACID SODIUM SALT / SODIUM FUSIDATE / FUSIDIC ACID SODIUM SALT 95.65View Details
751-94-0 -
 Sodium fusidate 95% CAS 751-94-0View Details
Sodium fusidate 95% CAS 751-94-0View Details
751-94-0 -
 Fusidic acid sodium salt CAS 751-94-0View Details
Fusidic acid sodium salt CAS 751-94-0View Details
751-94-0 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 CIS- BROMO BENZOATEView Details
CIS- BROMO BENZOATEView Details
61397-56-6 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
![1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
221615-75-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
