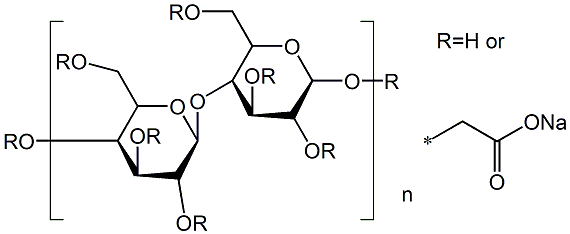
Sodium carboxyl methylstarch
Synonym(s):Carboxymethyl starch sodium salt;Sodium starch glycolate
- CAS NO.:9063-38-1
- Empirical Formula: C2H4O3·xNa·x
- Molecular Weight: 0
- MDL number: MFCD00217761
- EINECS: 618-597-7
- Update Date: 2025-12-17 09:49:15
What is Sodium carboxyl methylstarch?
Chemical properties
White or almost white, fine, free-flowing powder, very hygroscopic.
Chemical properties
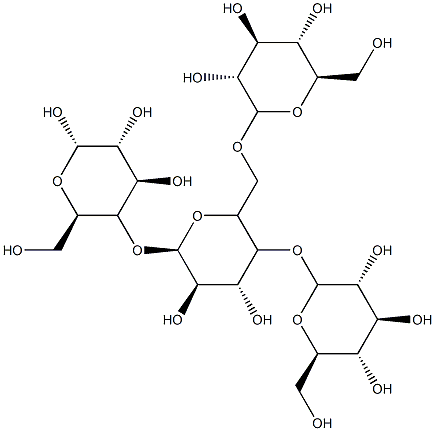
Sodium starch glycolate is a white or almost white free-flowing very hygroscopic powder. The PhEur 6.0 states that when examined under a microscope it is seen to consist of: granules, irregularly shaped, ovoid or pear-shaped, 30–100 mm in size, or rounded, 10–35 mm in size; compound granules consisting of 2–4 components occur occasionally; the granules have an eccentric hilum and clearly visible concentric striations. Between crossed nicol prisms, the granules show a distinct black cross intersecting at the hilum; small crystals are visible at the surface of the granules. The granules show considerable swelling in contact with water.
The Uses of Sodium carboxyl methylstarch
Sodium starch glycolate is widely used in oral pharmaceuticals as a disintegrant in capsule and tablet formulations. It is recommended to use in tablets prepared by either directcompression or wet-granulation processes.
The Uses of Sodium carboxyl methylstarch
Sodium Starch Glycolate is a starch of potato origin with α1-4 short linear linkages and branched α1-6 linkages between glucose units. Used in the synthesis of capsules for delivery of drugs or medicaments.
Production Methods
Sodium starch glycolate is a substituted derivative of potato starch.
Typically, commercial products are also crosslinked using either
sodium trimetaphosphate (Types A and B) or dehydration (Type
C).
Starch is carboxymethylated by reacting it with sodium
chloroacetate in an alkaline, nonaqueous medium, typically
denatured ethanol or methanol, followed by neutralization with
citric acid, acetic acid, or some other acid. Vivastar P is
manufactured in methanolic medium, and Explotab in ethanolic
medium.
Pharmaceutical Applications
Sodium starch glycolate is widely used in oral pharmaceuticals as a
disintegrant in capsule and tablet formulations. It is
commonly used in tablets prepared by either direct-compression or wet-granulation processes. The usual concentration
employed in a formulation is between 2% and 8%, with the
optimum concentration about 4%, although in many cases 2% is
sufficient. Disintegration occurs by rapid uptake of water followed
by rapid and enormous swelling.
Although the effectiveness of many disintegrants is affected by
the presence of hydrophobic excipients such as lubricants, the
disintegrant efficiency of sodium starch glycolate is unimpaired.
Increasing the tablet compression pressure also appears to have no
effect on disintegration time.
Sodium starch glycolate has also been investigated for use as a
suspending vehicle.
Safety
Sodium starch glycolate is widely used in oral pharmaceutical formulations and is generally regarded as a nontoxic and nonirritant material. However, oral ingestion of large quantities may be harmful.
Storage
Tablets prepared with sodium starch glycolate have good storage
properties. Sodium starch glycolate is stable although very
hygroscopic, and should be stored in a well-closed container in
order to protect it from wide variations of humidity and
temperature, which may cause caking.
The physical properties of sodium starch glycolate remain
unchanged for up to 3 years if it is stored at moderate temperatures
and humidity.
Incompatibilities
Sodium starch glycolate is incompatible with ascorbic acid.
Regulatory Status
Included in the FDA Inactive Ingredients Database (oral capsules and tablets). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Properties of Sodium carboxyl methylstarch
| Melting point: | >210°C (dec.) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | Practically insoluble in methylene chloride. It gives a translucent suspension in water. |
| form | neat |
| form | Solid |
| color | White to Off-White |
| CAS DataBase Reference | 9063-38-1 |
| EPA Substance Registry System | Starch, carboxymethyl ether, sodium salt (9063-38-1) |
Safety information for Sodium carboxyl methylstarch
Computed Descriptors for Sodium carboxyl methylstarch
Sodium carboxyl methylstarch manufacturer
Gangwal Healthcare Pvt Ltd
Attar Global
Vasa Pharmachem Pvt Ltd. (VPPL)
Chemoil Pharma
Shreeji Pharma International
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



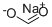
You may like
-
 Sodium starch glycollate CAS 9063-38-1View Details
Sodium starch glycollate CAS 9063-38-1View Details
9063-38-1 -
 Sodium Starch Glycolate ex. Maize (SSG) ExiPlus CAS 9063-38-1View Details
Sodium Starch Glycolate ex. Maize (SSG) ExiPlus CAS 9063-38-1View Details
9063-38-1 -
 Sodium carboxyl methylstarch CAS 9063-38-1View Details
Sodium carboxyl methylstarch CAS 9063-38-1View Details
9063-38-1 -
 Sodium Starch Glycolate, Grade Standard: pharma gradeeView Details
Sodium Starch Glycolate, Grade Standard: pharma gradeeView Details
9063-38-1 -
 Powder Sodium Starch Glycolate BPView Details
Powder Sodium Starch Glycolate BPView Details
9063-38-1 -
 Sodium Starch Glycolate Extra PureView Details
Sodium Starch Glycolate Extra PureView Details
9063-38-1 -
 Sodium Starch Glycolate, Grade Standard: IPView Details
Sodium Starch Glycolate, Grade Standard: IPView Details
9063-38-1 -
 Sodium Starch Glycollate - IP/BP/USP/EPView Details
Sodium Starch Glycollate - IP/BP/USP/EPView Details
9063-38-1
