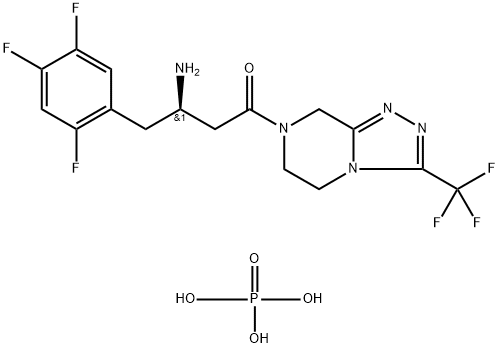
Sitagliptin phosphate
Synonym(s):7-[(3R)-3-amino-1-oxo-4-(2,4,5-trifluorophenyl)butyl]-5,6,7,8-tetrahydro-3-(trifluoromethyl)-1,2,4-triazolo[4,3-a]pyrazine phosphate;MK-0431;Sitagliptin phosphate monohydrate
- CAS NO.:654671-78-0
- Empirical Formula: C16H15F6N5O.H3PO4
- Molecular Weight: 505.31
- MDL number: MFCD09952339
- EINECS: 1806241-263-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-11 08:41:34
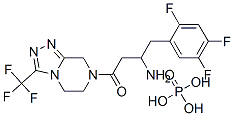
What is Sitagliptin phosphate?
Description
Sitagliptin is the first novel dipeptidyl peptidase IV inhibitor from Merck for the treatment of type 2 diabetes without weight gain and the incidence of hypoglycemia was similar to placebo. Sitagliptin acts by enhancing the body’s incretin system, which helps to regulate glucose by affecting β and α cells in the pancreas.
Chemical properties
White Solid
The Uses of Sitagliptin phosphate
A trizolopyrazine dipeptidyl peptidase IV inhibitor. It has recently been approved for the therapy of type II diabetes.
The Uses of Sitagliptin phosphate
Sitagliptin is a trizolopyrazine dipeptidyl peptidase IV inhibitor. Sitagliptin has recently been approved for the therapy of type II diabetes.
What are the applications of Application
Sitagliptin Phosphate is a trizolopyrazine dipeptidyl peptidase IV inhibitor
General Description
Sitagliptin phosphate is the 1:1 phosphoric acid salt ofsitagliptin free base (i.e., (2R)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl]-1-(2,4,5-trifluorophenyl)-2-butanamine), and is marketed(Junuvia, 2006) in 25-, 50-, and 100-mg tablets. Acombination product with metformin (Janumet, 2007) intwo strengths (50 mg/500 mg and 50 mg/1,000 mgsitagliptin/metformin) is also available, and sitagliptin mayalso be prescribed with a thiazolidinedione, or possibly a sulfonylurea.The phosphate salt provides very high water solubility.The bioavailability of orally administered sitagliptinis~87%. The drug exhibits relatively low plasma protein binding (~38%), a relatively large volume of distribution(198 L), and a terminal elimination half-life of 12 hours.About 79% of a 100-mg oral dose is excreted unchanged inurine, the balance as trace-level metabolites (CYP3A4, lessercontribution by CYP2C8) in urine or feces: 87% of administeredradioactivity is excreted in urine, and 13% in feces.Active tubular excretion is reported to play a key role in renalclearance of unchanged drug, and may be mediated at least inpart via the organic anion transporter hOAT-3.
Synthesis
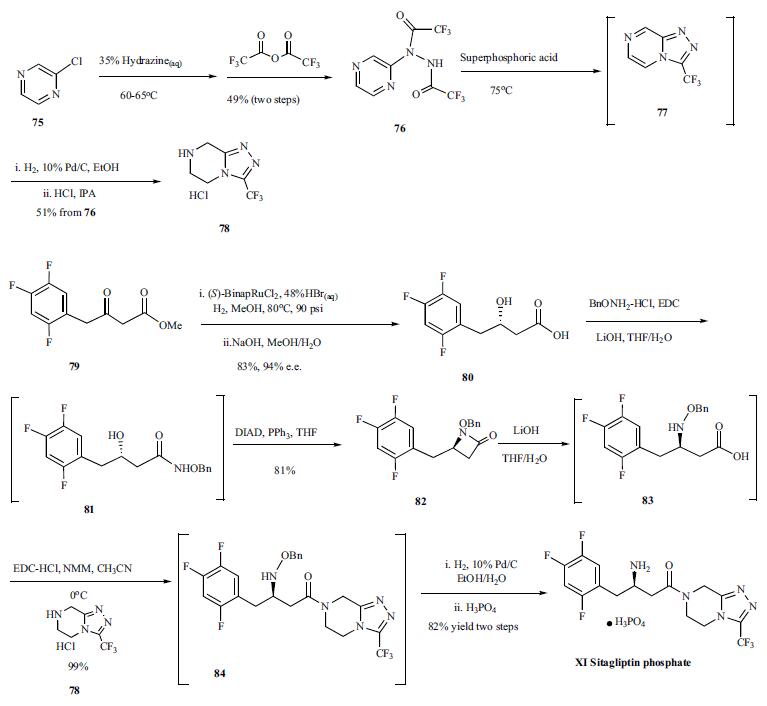
Synthesis of sitagliptin started with the slow addition of chloropyrazine (75) to 35% aqueous hydrazine at 60-65??C, controlling this exothermic reaction and making it process-friendly, and the resulting crude pyrazinyl hydrazine was acetylated with trifluoroacetic anhydride to afford bis-trifluoromethylhydrazide 76 in 49% yield from the chloropyrazine. Compound 76 was treated with superphosphoric acid, a diluted form of polyphosphoric acid, to give cyclized compound 77 which was hydrogenated with Pd/C and the resulting product was treated with HCl in IPA to afford compound 78 as its HCl salt in 51% yield from 76. Compound 78 was used later on in a coupling reaction to generate sitagliptin. Compound 79, a beta-ketoester, was subjected to asymmetric reduction with (S)-BinapRuCl2-triethylamine complex in methanol at 80??C, catalytic amount of hydrogen bromide, and 90 psi of hydrogen atmosphere to give the desired beta-hydroxy ester which was hydrolyzed to give carboxylic acid 80 in 94% e.e. and 83% yield. The carboxylic acid 80 was coupled with BnONH2-HCl in the presence of EDC and lithium hydroxide in THF/H2O to give coupled compound 81 which was cyclized to compound 82 with DIAD and triphenylphosphine in THF in 81% yield from compound 80. Compound 82 was then hydrolyzed to |?-amino acid 83 with lithium hydroxide, and the acid was coupled with compound 78 at 0??C with EDC-HCl and NMM as base to give compound 84 in excellent yield. Compound 84 was hydrogenated with 10% Pd/C in an ethanol/H2O mix solvent system. The water was crucial to complete the reaction and restore catalyst activity. Finally, the ethanol solution of the hydrogenated product was treated with phosphoric acid, and sitagliptin (XI) was crystallized as its anhydrous phosphoric acid salt from aqueous ethanol solution.
in vitro
sitagliptin was a potent inhibitor for dpp-4 with an ic50 of 18 nm [1]. sitagliptin inhibited dpp-8 with an ic50 of 48 μm. sitagliptin showed no effect on several related peptidases, including dpp-9, dpp-ii, and amino peptidase p [1].
Storage
Desiccate at RT
References
[1] biftu t, feng d, qian x, et al. (3r)-4-[(3r)-3-amino-4-(2, 4, 5-trifluorophenyl) butanoyl]-3-(2, 2, 2-trifluoroethyl)-1, 4-diazepan-2-one, a selective dipeptidyl peptidase iv inhibitor for the treatment of type 2 diabetes[j]. bioorganic & medicinal chemistry letters, 2007, 17(1): 49-52.
[2] fleischer b. cd26: a surface protease involved in t-cell activation[j]. immunology today, 1994, 15(4): 180-184.
[3] aschner p, kipnes m s, lunceford j k, et al. effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes[j]. diabetes care, 2006, 29(12): 2632-2637.
[4] green j b, bethel m a, armstrong p w, et al. effect of sitagliptin on cardiovascular outcomes in type 2 diabetes[j]. new england journal of medicine, 2015, 373(3): 232-242.
Properties of Sitagliptin phosphate
| Melting point: | 202-204°C |
| storage temp. | -20°C Freezer |
| solubility | Methanol (Slightly, Heated), Water (Sparingly, Sonicated) |
| form | Solid |
| color | White to Off-White |
| InChI | InChI=1S/C16H15F6N5O.H3O4P/c17-10-6-12(19)11(18)4-8(10)3-9(23)5-14(28)26-1-2-27-13(7-26)24-25-15(27)16(20,21)22;1-5(2,3)4/h4,6,9H,1-3,5,7,23H2;(H3,1,2,3,4) |
Safety information for Sitagliptin phosphate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Sitagliptin phosphate
| InChIKey | IQFYVLUXQXSJJN-UHFFFAOYSA-N |
| SMILES | C(C1=CC(F)=C(F)C=C1F)C(N)CC(=O)N1CCN2C(C(F)(F)F)=NN=C2C1.P(O)(O)(O)=O |
Sitagliptin phosphate manufacturer
DR ACHARYA LABORATORIES PVT LTD
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
![3-(Trifluoromethyl)-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazine hydrochloride](https://img.chemicalbook.in/CAS/GIF/762240-92-6.gif)

![Benzenebutanoicacid,b-[[(1,1-diMethylethoxy)carbonyl]aMino]-2,4,5-trifluoro-,Methylester,(bR)-](https://img.chemicalbook.in/CAS/20150408/GIF/881995-73-9.gif)
You may like
-
 654671-78-0 98%View Details
654671-78-0 98%View Details
654671-78-0 -
 Sitagliptin Phosphate (Monohydrate and Anhydrous) 98%View Details
Sitagliptin Phosphate (Monohydrate and Anhydrous) 98%View Details
654671-78-0 -
 Sitagliptin Phosphate 99%View Details
Sitagliptin Phosphate 99%View Details -
 654671-78-0 Sitagliptin Phosphate 98%View Details
654671-78-0 Sitagliptin Phosphate 98%View Details
654671-78-0 -
 654671-78-0 99%View Details
654671-78-0 99%View Details
654671-78-0 -
 Sitagliptin phosphate 98% (HPLC) CAS 654671-78-0View Details
Sitagliptin phosphate 98% (HPLC) CAS 654671-78-0View Details
654671-78-0 -
 Sitagliptin Phosphate APIView Details
Sitagliptin Phosphate APIView Details
486460-32-6 -
 Api Powder Sitagliptin Phosphate, Grade Standard: USPView Details
Api Powder Sitagliptin Phosphate, Grade Standard: USPView Details
654671-77-9
