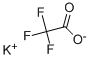
Silver trifluoroacetate
Synonym(s):Silver trifluoroacetate;Trifluoroacetic acid silver salt
- CAS NO.:2966-50-9
- Empirical Formula: C2AgF3O2
- Molecular Weight: 220.88
- MDL number: MFCD00013199
- EINECS: 221-004-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30

What is Silver trifluoroacetate?
Chemical properties
light beige to grey crystalline powder
The Uses of Silver trifluoroacetate
Silver Trifluoroacetate functions as a catalyst in the synthesis of cyclobutene-fused azepines/dihydropyridines.
What are the applications of Application
Silver trifluoroacetate is a Lewis acid catalyst
What are the applications of Application
Silver trifluoroacetate is the silver salt of trifluoroacetic acid and is used as a Lewis acid catalyst. It is used as an important raw material for the preparation of triethylsilyl trifloroacetate and triphenylmethyl trifluoroacetate. Further, it is used as a catalyst in the preparation of 4-iodoveratrole from veratrole by reacting with iodine.
Synthesis
Silver trifluoroacetate is prepared by the reaction of silver oxide and trifluoroacetic acid.
Reaction equation: Ag2O+2CF3COOH→2CF3COOAg+H2O
Reaction: 187g (0.81mol) of silver oxide was weighed and suspended in 200mL of water, and 177g (1.55mol) of trifluoroacetic acid was added. After filtration, the filtrate was evaporated to dryness under reduced pressure. The residue was extracted with ether in a Soxhlet extractor. Alternatively, dissolve the residue in 1.2L of ether and filter through a thin layer of activated carbon. After distilling off the ether, 300g of the product was obtained with a yield of 83%.
Purification Methods
The extract is filtered and evaporated to dryness, then the powdered residue is completely dried in a vacuum desiccator over silica gel. Its solubility in Et2O is 33.5g in 750mL. It can be recrystallised from *C6H6 (solubility is: 1.9g in 30mL of *C6H6, and 33.5g will dissolve in 750mL of anhydrous Et2O). [Traynham & Dehn J Org Chem 23 1545 1958, Haszeldine J Chem Soc 584 1951.] Store it in the dark. It is also soluble in trifluoroacetic acid (15.2% at 30o), toluene, o-xylene and dioxane [Hara & Cady J Am Chem Soc 76 4285 1954]. [Beilstein 2 IV 461.]
Properties of Silver trifluoroacetate
| Melting point: | 257-260 °C (dec.) (lit.) |
| storage temp. | Store below +30°C. |
| form | Crystalline Powder |
| color | Light beige to gray |
| Water Solubility | SOLUBLE |
| Sensitive | Hygroscopic |
| BRN | 3634022 |
| CAS DataBase Reference | 2966-50-9(CAS DataBase Reference) |
| EPA Substance Registry System | Acetic acid, trifluoro-, silver(1+) salt (2966-50-9) |
Safety information for Silver trifluoroacetate
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H300:Acute toxicity,oral H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. |
Computed Descriptors for Silver trifluoroacetate
Silver trifluoroacetate manufacturer
JSK Chemicals
Dhara Industries
ARRAKIS INDUSTRIES LLP
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 silver trifluoroacetate 99%View Details
silver trifluoroacetate 99%View Details -
 Silver trifluoroacetate CAS 2966-50-9View Details
Silver trifluoroacetate CAS 2966-50-9View Details
2966-50-9 -
 Silver trifluoroacetate CAS 2966-50-9View Details
Silver trifluoroacetate CAS 2966-50-9View Details
2966-50-9 -
 Silver trifluoroacetate CAS 2966-50-9View Details
Silver trifluoroacetate CAS 2966-50-9View Details
2966-50-9 -
 Silver trifluoroacetate 95.00% CAS 2966-50-9View Details
Silver trifluoroacetate 95.00% CAS 2966-50-9View Details
2966-50-9 -
 Silver trifluoroacetate 98% CAS 2966-50-9View Details
Silver trifluoroacetate 98% CAS 2966-50-9View Details
2966-50-9 -
 Silver trifluoroacetate 98.00% CAS 2966-50-9View Details
Silver trifluoroacetate 98.00% CAS 2966-50-9View Details
2966-50-9 -
 Trifluoroacetic acid CAS 2966-50-9View Details
Trifluoroacetic acid CAS 2966-50-9View Details
2966-50-9