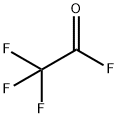
Trifluoroacetyl chloride
- CAS NO.:354-32-5
- Empirical Formula: C2ClF3O
- Molecular Weight: 132.47
- MDL number: MFCD00039307
- EINECS: 206-556-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
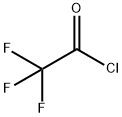
What is Trifluoroacetyl chloride?
The Uses of Trifluoroacetyl chloride
Widely used in medicine, pesticides, organic intermediate and fine chemical.
Definition
ChEBI: Trifluoroacetyl chloride is an acyl chloride. It is functionally related to a trifluoroacetic acid.
General Description
A colorless gas. Shipped as a liquid under own vapor pressure. Contact with the unconfined liquid may frostbite unprotected skin. Very toxic by inhalation and may severely irritate skin, eyes, and mucous membranes. Under prolonged exposure to fire or heat the containers may rupture violently and rocket.
Air & Water Reactions
Reacts avidly with water and with moisture in the air to give fumes of hydrogen chloride, a water-soluble toxic gas.
Reactivity Profile
TRIFLUOROACETYL CHLORIDE is incompatible with strong oxidizing agents, alcohols, amines, alkalis. Reacts vigorously with amines and alkalis. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].
Health Hazard
TOXIC; may be fatal if inhaled, ingested or absorbed through skin. Vapors are extremely irritating and corrosive. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution.
Fire Hazard
Some may burn but none ignite readily. Vapors from liquefied gas are initially heavier than air and spread along ground. Some of these materials may react violently with water. Cylinders exposed to fire may vent and release toxic and/or corrosive gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket.
Flammability and Explosibility
Not classified
Safety Profile
Corrosive to skin, eyes, and materials. When heated to decomposition it emits very toxic fumes of Fand Cl-. See also FLUORIDES and CHLORIDES.
Properties of Trifluoroacetyl chloride
| Melting point: | −146 °C(lit.) |
| Boiling point: | −27 °C(lit.) |
| Density | 1,335 g/cm3 |
| vapor density | 4.6 (vs air) |
| vapor pressure | 546.6kPa at 25℃ |
| Flash point: | -58.1±25.9℃ |
| BRN | 1098994 |
| CAS DataBase Reference | 354-32-5(CAS DataBase Reference) |
| EPA Substance Registry System | Trifluoroacetyl chloride (354-32-5) |
Safety information for Trifluoroacetyl chloride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Trifluoroacetyl chloride
Trifluoroacetyl chloride manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Trifluoro acetylchloride 98%View Details
Trifluoro acetylchloride 98%View Details -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1