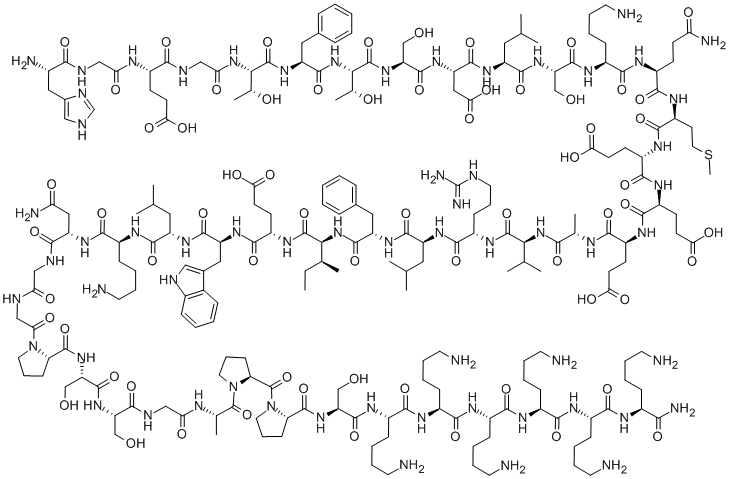
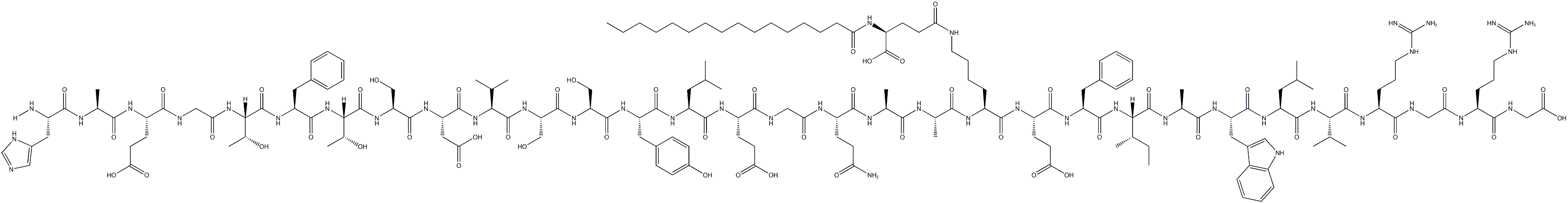
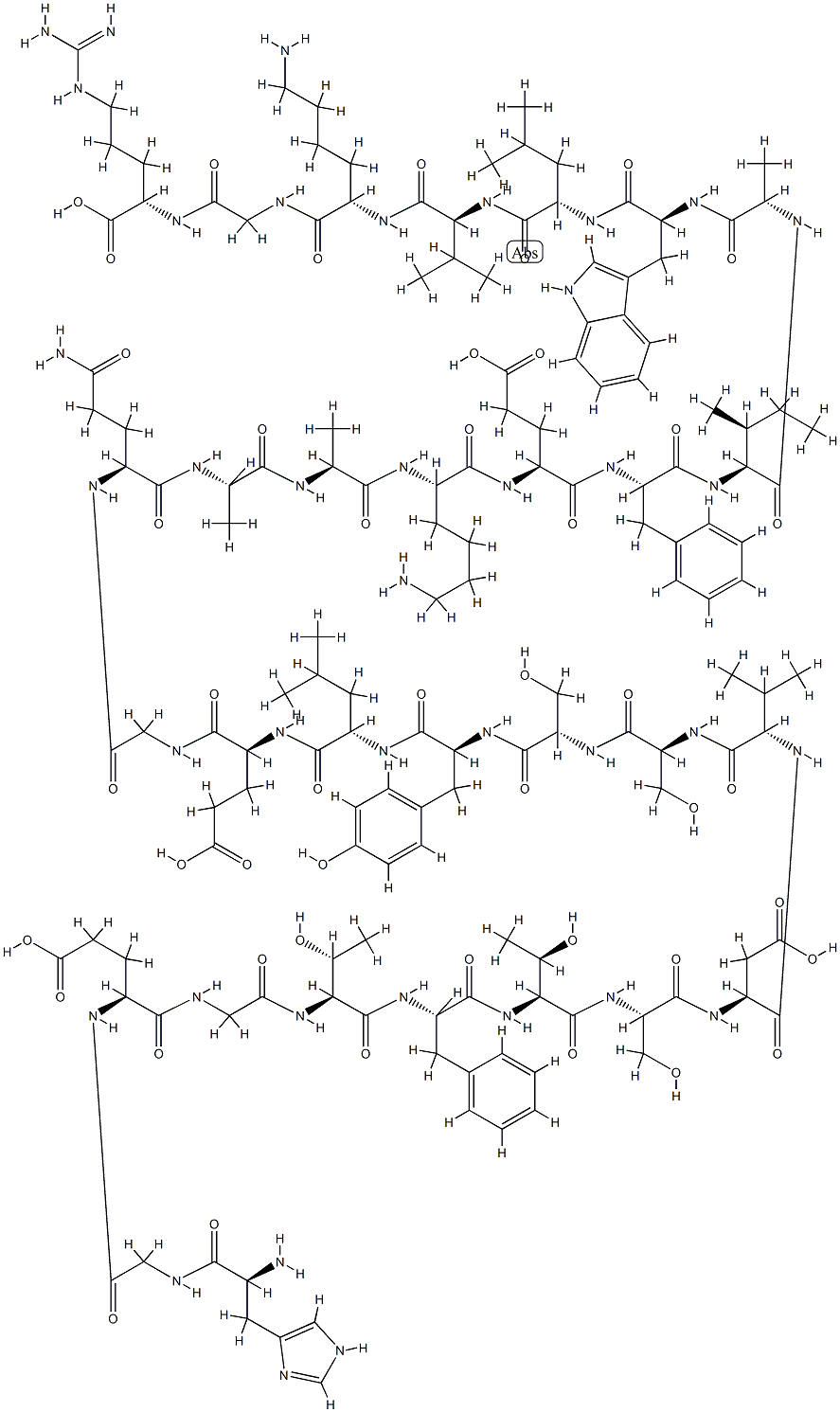
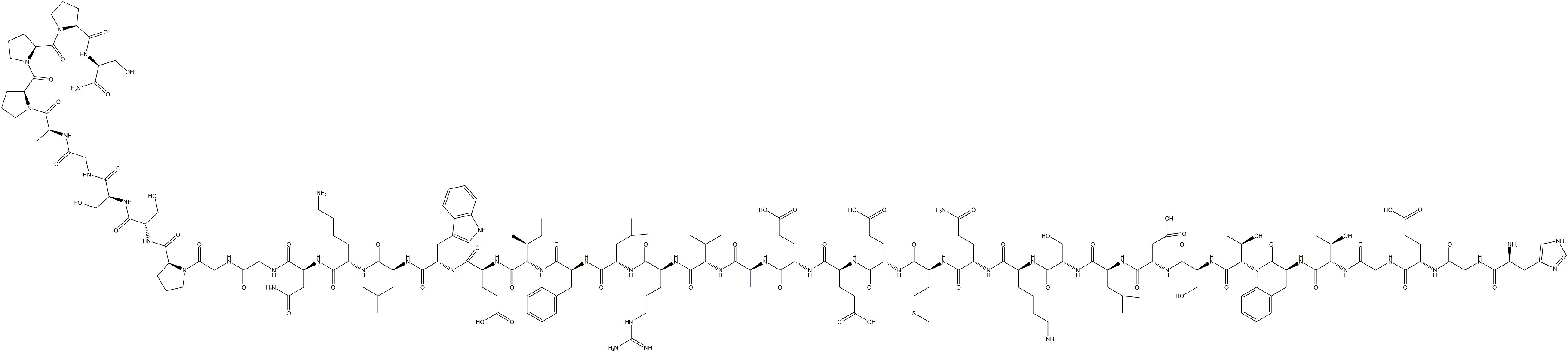
Semaglutide
- CAS NO.:910463-68-2
- Empirical Formula: C187H291N45O59
- Molecular Weight: 4113.57754
- EINECS: 203-405-2
- Update Date: 2024-12-23 19:56:12
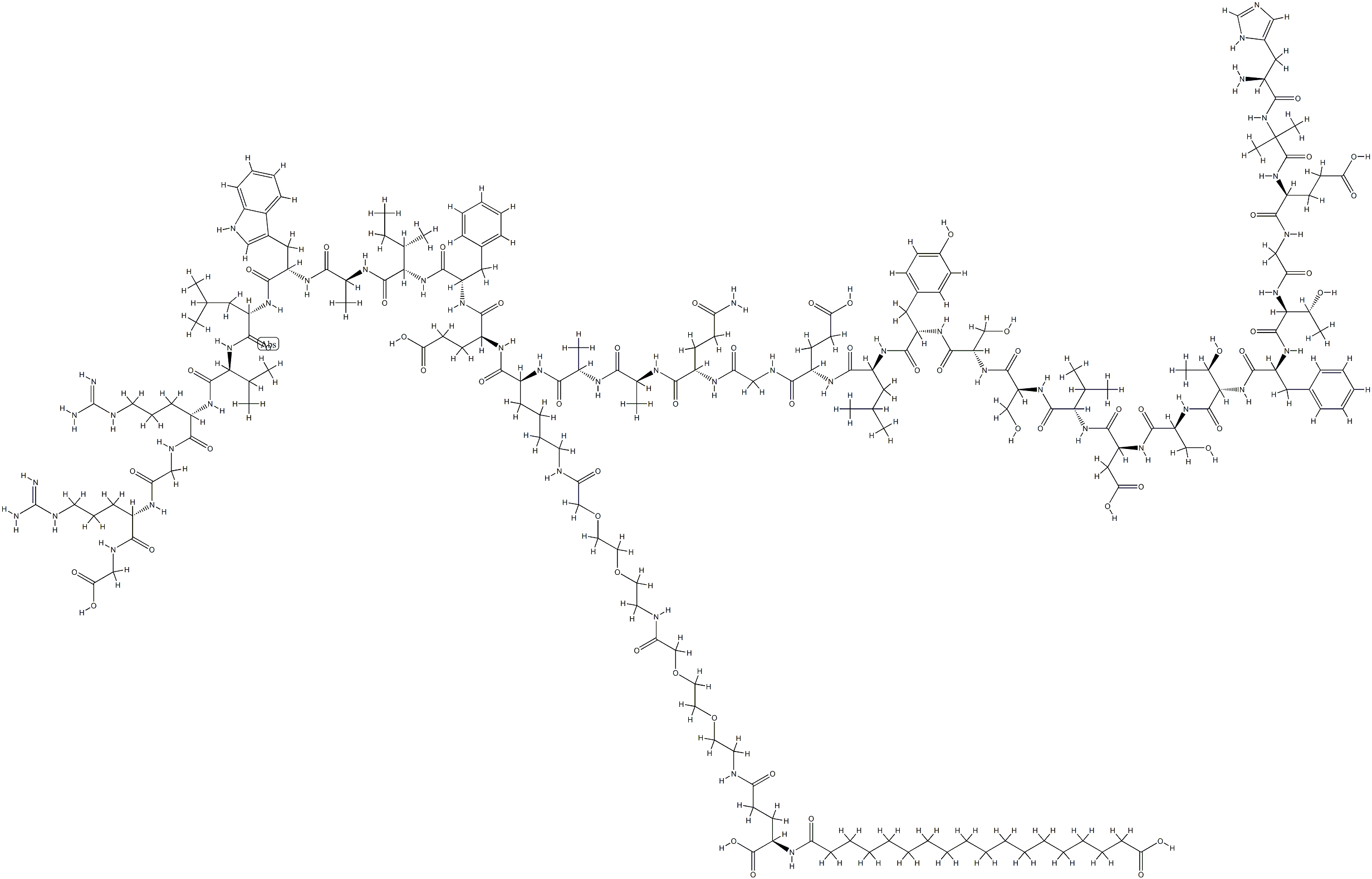
What is Semaglutide?
Absorption
The Cmax of semaglutide was 10.9 nmol/L, with AUC of 3123.4 nmol h/L and a Tmax of 56 h in one clinical trial, achieved within 1-3 days. The absolute bioavailability is 89%. Steady-state concentration of the oral tablet is achieved in 4-5 weeks. Average steady state concentrations of semaglutide are the mean steady state concentrations after dosing at 0.5mg to 1mg range from 16 nmol/L to 30 nmol/L.
Toxicity
Overdoses of up to 4 mg in one ingestion have been reported, with nausea being the most commonly reported symptom. All patients in clinical trials who experienced an overdose recovered fully. Appropriate supportive care should be given according and dictated by the patient's condition. Prolonged observation and treatment may be required, as the half-life of this drug is about one week. There is no antidote to an overdose with semaglutide.
Description
Semaglutide, sold under the brand name Ozempic among others, is an anti-diabetic medication used for the treatment of type 2 diabetes and chronic weight management. Semaglutide acts like human glucagon-like peptide-1 (GLP-1) such that it increases insulin secretion, thereby increasing sugar metabolism. It is distributed as a metered subcutaneous injection in a prefilled pen, or as an oral form. One of its advantages over other antidiabetic drugs is that it has a long duration of action, thus, only once-a-week injection is sufficient.
The Uses of Semaglutide
Semaglutide injection (Wegovy) is used along with an individualized low-calorie, low-fat diet and exercise program to help with weight loss in overweight adults who may also have high blood pressure, diabetes, or high cholesterol. Semaglutide injection is in a class of medications called incretin mimetics. It works by helping the pancreas to release the right amount of insulin when blood sugar levels are high. Insulin helps move sugar from the blood into other body tissues where it is used for energy. Semaglutide injection also works by slowing the movement of food through the stomach and may decrease appetite and cause weight loss.
Background
Semaglutide is a glucagon-like peptide 1 (GLP-1) analog used to manage type 2 diabetes along with lifestyle changes, such as dietary restrictions and increased physical activity. Other members of this drug class include Exenatide and Liraglutide. Semaglutide was developed by Novo Nordisk and approved by the FDA for subcutaneous injection in December 2017. The tablet formulation was approved for oral administration in September 2019. Semaglutide works by binding to and activating the GLP-1 receptor, thereby stimulating insulin secretion and reducing blood glucose.
The subcutaneous injection is administered once weekly and the tablet is administered once a day. Semaglutide offers a competitive advantage over other drugs used to manage diabetes, which may require several daily doses. Clinical trials have determined that this drug reduces glycosylated hemoglobin (HbA1c) levels and reduces body weight, proving to be effective for patients with type 2 diabetes. In June 2021, semaglutide was approved by the FDA for chronic weight management in adults with general obesity or overweight who have at least one weight-related condition, marking semaglutide as the first approved drug for such use since 2014. The use of semaglutide in weight management is also approved by Health Canada and the EMA.
On May 31, 2023, the FDA issued a warning regarding the use of compounded semaglutide after receiving adverse event reports. The use of salt forms of semaglutide, including semaglutide sodium and semaglutide acetate, has not been proven to be safe or effective.
Indications
Semaglutide is indicated to improve glycemic control in adults diagnosed with type 2 diabetes mellitus, and is used as an adjunct to diet and exercise. However, semaglutide is not a suitable first-line drug for diabetes that has not been controlled by diet and exercise. In addition, it has not been studied in patients with pancreatitis. Semaglutide is not intended for use in patients with type 1 diabetes or to treat diabetic ketoacidosis.
Semaglutide is indicated for chronic weight management in adults with obesity or overweight with at least one weight-related condition (such as high blood pressure, type 2 diabetes, or high cholesterol), for use in addition to a reduced-calorie diet and increased physical activity.. Semaglutide it is also indicated for chronic weight management in pediatric patients aged 12 years and older with an initial BMI at the 95th percentile or greater for age and sex.
Definition
ChEBI: Semaglutide is a polypeptide that contains a linear sequence of 31 amino acids joined together by peptide linkages. It is an agonist of glucagon-like peptide-1 receptors (GLP-1 AR) and used for the treatment of type 2 diabetes. It has a role as a hypoglycemic agent, a glucagon-like peptide-1 receptor agonist, an anti-obesity agent, a neuroprotective agent and an appetite depressant. It is a polypeptide and a lipopeptide.
Biological Activity
Semaglutide (Rybelsus, Ozempic, NN9535, OG217SC, NNC 0113-0217), a long-acting glucagon-like peptide 1 (GLP-1) analogue, is a GLP-1 receptor agonist with the potential for the treatment of type 2 diabetes mellitus (T2DM).
Mechanism of action
Semaglutide is a glucagon-like peptide-1 receptor agonist. It increases the production of insulin, a hormone that lowers the blood sugar level. It also appears to enhance growth of β cells in the pancreas, which are the sites of insulin production. It also inhibits glucagon, which is a hormone that increases blood sugar. It additionally reduces food intake by lowering appetite and slows down digestion in the stomach. In this way it reduces body fat.
Pharmacokinetics
Semaglutide reduces HbA1c, systolic blood pressure, and body weight. After 12 weeks of treatment, semaglutide decreased fasting and postprandial glucose by increasing insulin production and decreasing glucagon secretion (which is normally associated with increases in blood sugar). Semaglutide also lowers fasting triglycerides and VLDL cholesterol, exerting beneficial effects on cardiovascular health.
Semaglutide has been shown to cause medullary thyroid cell carcinoma in rodents. While its clinical relevance to humans is unknown, the FDA advises not to administer this drug in those with a personal or family history of medullary thyroid carcinoma. Semaglutide also poses a risk of pancreatitis and dehydration. Patients must be adequately hydrated while on semaglutide and are advised to seek medical attention immediately in cases of abdominal pain radiating to the back. Because this drug delays gastric emptying, it is important to monitor for the efficacy or adverse effects of other drugs that are administered orally.
Pharmacokinetics
Median tmax,semaglutide was 1.5 hours for both water volumes with a range of 0.5-3.0 hours for 50 mL and a range of 0.5-4.0 hours for 240 mL. AUC0-24h,semaglutide and Cmax,semaglutide were approximately 70% higher when dosed with 50 versus 240 mL water[2].
Side Effects
Some common side effects of Semaglutide include: nausea, vomiting, diarrhea, abdominal pain, and constipation may occur.
Less common but serious side effects of Semaglutide include: kidney problems, diabetic retinopathy, allergic reactions, low blood sugar, and pancreatitis.
In people with heart problems, it can cause damage to the back of the eye (retinopathy). If you experience any of these serious side effects, contact your healthcare provider immediately.
www.mayoclinic.org
in vitro
Semaglutide is selected as the optimal once weekly candidate. Semaglutide has two amino acid substitutions compared to human GLP-1 (Aib8, Arg34) and is derivatized at lysine 26. The GLP-1R affinity of semaglutide (0.38 ± 0.06 nM) is three-fold decreased compared to liraglutide, whereas the albumin affinity is increased.
in vivo
The plasma half-life is 46.1 h in mini-pigs following i.v. administration, and semaglutide has an MRT of 63.6 h after s.c. dosing to mini-pigs.
Metabolism
Semaglutide is cleaved at the peptide backbone, followed by β‐oxidation of the fatty acid chain. Naturally occurring GLP‐1 is quickly metabolized by dipeptidyl peptidase‐4 (DPP‐4) and other enzymes, which is ubiquitous in human tissues. Chemical structure modifications render semaglutide less susceptible to enzymatic degradation by gastrointestinal DPP‐4 enzymes. It is slowly and extensively metabolized, with about 83% of the administered dose measured in the plasma as unchanged drug. Neural endopeptidase (NEP) is another enzyme that metabolizes this drug. DPP-4 inactivates semaglutide, truncating the N-terminal segment while NEP hydrolyzes peptide bondsSix different metabolites of semaglutide have been identified in human plasma. The major metabolite, named P3, accounts for about 7.7% of an ingested dose.
Metabolism
Intact semaglutide was the primary component circulating in plasma for humans and both nonclinical species, accounting for 69–83% of the total amount of semaglutide-related material, and was metabolised prior to excretion. Recovery of excreted radioactivity was 75.1% in humans, 72.1% in rats and 58.2% in monkeys. Urine and faeces were shown to be important routes of excretion, with urine as the primary route in both humans and animals[1]. Semaglutide was metabolised through proteolytic cleavage of the peptide backbone and sequential beta-oxidation of the fatty acid sidechain, and metabolism was not confined to specific organs. Intact semaglutide in urine accounted for 3.1% of the administered dose in humans and less than 1% in rats; it was not detected in urine in monkeys.
Precautions
The FDA recommends Wegovy for weight loss if you meet one of the following criteria: Have a body mass index (BMI) of 27kg/m2 or greater and at least one weight-related condition, such as high blood pressure, Type 2 diabetes, or high cholesterol; Have a BMI of 30kg/m2 or greater.
Avoid semaglutide if you have: History of medullary thyroid cancer; History of gallbladder disease; History of pancreatitis; Multiple endocrine neoplasia syndrome type 2 (MEN2).
References
[1] A, Lene Jensen , et al. "Absorption, metabolism and excretion of the GLP-1 analogue semaglutide in humans and nonclinical species." European Journal of Pharmaceutical Sciences 104(2017):31-41.
[2] Tine A. B?kdal. “Relationship Between Oral Semaglutide Tablet Erosion and Pharmacokinetics: A Pharmacoscintigraphic Study.” Clinical Pharmacology in Drug Development 10 5 (2021): 453–462.
Properties of Semaglutide
| storage temp. | Store at -20°C |
| solubility | DMSO:3mg/mL (0.73 mM) |
| form | Solid |
| color | White to off-white |
Safety information for Semaglutide
Computed Descriptors for Semaglutide
| InChIKey | DLSWIYLPEUIQAV-CCUURXOWSA-N |
Semaglutide manufacturer
Meenaakshi Molecules Pvt Ltd
Anthem Biosciences Pvt Ltd
Shilpa Medicare Limited (SML)
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 910463-68-2 Semaglutide 98%View Details
910463-68-2 Semaglutide 98%View Details
910463-68-2 -
 Semaglutide 98%View Details
Semaglutide 98%View Details -
 910463-68-2 99%View Details
910463-68-2 99%View Details
910463-68-2 -
 Semaglutide 910463-68-2 / 910463-93-3 99%View Details
Semaglutide 910463-68-2 / 910463-93-3 99%View Details
910463-68-2 / 910463-93-3 -
 910463-68-2 / 910463-93-3 Semaglutide 99%View Details
910463-68-2 / 910463-93-3 Semaglutide 99%View Details
910463-68-2 / 910463-93-3 -
 910463-68-2 / 910463-93-3 98%View Details
910463-68-2 / 910463-93-3 98%View Details
910463-68-2 / 910463-93-3 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8