Selenium Oxychloride
Synonym(s):Seleninyl chloride
- CAS NO.:7791-23-3
- Empirical Formula: Cl2OSe
- Molecular Weight: 165.87
- MDL number: MFCD00016327
- EINECS: 232-244-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
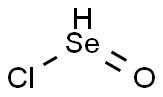
What is Selenium Oxychloride?
Chemical properties
Selenium oxychloride is a colorless to yellowish liquid. Fumes in air.
Physical properties
Pale yellow or colorless liquid; corrosive; refractive index 1.651 at 20°C; density 2.42 g/mL at 22°C; freezes at 8.5°C; boils at 176.4°C; decomposes at 176.4°C; decomposes in water forming hydrochloric acid and selenious acid; soluble in carbon disulfide, carbon tetrachloride, chloroform, benzene, and toluene.
The Uses of Selenium Oxychloride
selenium oxychloride is used as solvent.
The Uses of Selenium Oxychloride
Selenium(IV) chloride oxide is used as a polar solvent for synthetic phenolic resins. It is also used to prepare selenium oxybromide and selenium nitride. It acts as a chlorinating agent.
What are the applications of Application
Selenium oxychloride is a very desirable solvent as it has a high dielectric constant and high specific conductance
Production Methods
Selenium oxychloride (SeOCl2) is a powerful solvent, chlorinating agent, and resin plasticizer used in the chemical industry.
General Description
A colorless to light-colored liquid. Insoluble in water and denser than water. Contact may severely irritate skin, eyes and mucous membranes. May be toxic by ingestion, inhalation and skin absorption. Used to make other chemicals.
Air & Water Reactions
Fumes in air. Insoluble in water and denser than water. Decomposed in water or moist air to form hydrochloric acid and selenious acid [Merck 11th ed. 1989].
Reactivity Profile
Red phosphorus reacts in the cold with SELENIUM OXYCHLORIDE evolving light and heat; white phosphorus reacts explosively [Mellor 10:906 1946-47]. When potassium is brought into contact with SELENIUM OXYCHLORIDE in the cold, an explosion occurs [Mellor 10:908 1946-47]. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291]. Powdered antimony ignites on contact with the chloride, [Mellor, 1947, Vol. 10, 906]. With metal oxides, i.e. silver oxide, light is evolved and heat sufficient to decompose the mixture, [Mellor, 1947, Vol. 10, 909].
Hazard
Extremely hazardous, toxic irritant, poison, severe vesicant.
Health Hazard
SELENIUM OXYCHLORIDE is very toxic and may cause death or permanent injury after very short exposures to small quantities. Inhalation of small quantities may be corrosive and irritating to the respiratory tract. It can burn and irritate the skin and eyes and cause burns when ingested. Long-term exposure to selenium compounds may be a cause of amyotrophic lateral sclerosis in humans. Populations at special risk include those with a history of dermatitis, chronic bronchitis, skin allergies, respiratory tract infections, liver or kidney disease, jaundice, or albuminuria. Women of child-bearing age are also considered at risk.
Fire Hazard
When SELENIUM OXYCHLORIDE is heated to decomposition, or in contact with acids or acid fumes, highly toxic chloride and selenium fumes are evolved. Hydrochloric acid and selenious acid are produced by reaction with water. Decomposed by water. Reacts violently with powdered antimony, red and white phosphorus, disilver oxide, lead oxides, and potassium. Avoid water, moist air.
Safety Profile
Poison by skin contact and subcutaneous routes. Human systemic effects by skin contact with very small amounts: primary irritant, corrosive. Explodes on contact with potassium, white phosphorus. Ignites on contact with antimony. Vigorous reaction with metal oxides (e.g., silver oxide, lead@) oxide, lead(Ⅳ) oxide, lead(II)(IV) oxide). When heated to decomposition it emits very toxic fumes of Cland Se. See also SELENIUM COMPOUNDS and CHLORIDES.
Potential Exposure
This material is used as a solvent for many substances, including metals and as a chlorinating agent; and resin plasticizer; as an ionizing solvent; for monochlorination of ketones.
Shipping
UN2879 Selenium oxychloride, Hazard class: 8; Labels: 8-Corrosive material, 6.1-Poisonous material.
Incompatibilities
Water and air reactive releasing strong acids. The aqueous solution is a strong acid and oxidizer.Reacts violently with bases, reducing agents, hydrides, powdered antimony, red, and white phosphorus, disilver oxide, lead oxide; powdered metals, and potassium. Note: Never pour water into this substance; when dissolving or diluting always add it slowly to the water
Properties of Selenium Oxychloride
| Melting point: | 9 °C |
| Boiling point: | 180 °C(lit.) |
| Density | 2.43 g/mL at 25 °C(lit.) |
| refractive index | 1.651 |
| Flash point: | 176.4°C |
| solubility | Miscible with chloroform, carbon disulphide and benzene. |
| form | Liquid |
| color | Yellow |
| Specific Gravity | 2.42 |
| Water Solubility | decomposed in H2O to HCl, selenious acid; miscible with CCl4, CHCl3, CS2, benzene, toluene [MER06] |
| Sensitive | Moisture Sensitive |
| Merck | 14,8435 |
| Exposure limits | ACGIH: TWA 0.2 mg/m3 NIOSH: IDLH 1 mg/m3; TWA 0.2 mg/m3 |
| Dielectric constant | 46.0 |
| Stability: | Stable, but reacts with water and moisture. Incompatible with water, potassium, phosphorus. |
| CAS DataBase Reference | 7791-23-3(CAS DataBase Reference) |
| EPA Substance Registry System | Selenium oxychloride (7791-23-3) |
Safety information for Selenium Oxychloride
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H373:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P314:Get medical advice/attention if you feel unwell. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Selenium Oxychloride
New Products
3-pyridine carboxaldehyde 7-bromoquinoxalin-2(1H)-one 8-Bromoisoquinoline 2-Bromo-6-flurobenzaldehyde 3-Pyridineacetonitrile, α-amino-, hydrochloride (1:1) Trans-methyl 4-aminocyclohexane- carboxylate HCl N-Boc-L-Alaninol 4-[2-(DIMETHYLAMINO)ETHOXY]BENZYLAMINE Ibanic Acid Suzetrigine 5,6-Dichloronicotinic acid 2-(2-chloroethoxy)-5-nitrobenzaldehyde Remibrutinib Dimethyl 4-aminothiophene-2,3-dicarboxylate hydrochloride 1H-Indole-1-carboxylic acid, 5-hydroxy-7-methyl-, 1,1-dimethylethyl ester N N N'Trimethyl ethylenediamine Variamine Blue B Diazonium salt Lead II Bromide N N' DimethylEthylenediamine Ethyl Methanesulfonate N Ethylmethylamine Radiator Flux Zinc Chloride Solution (All Grades) H-Ser(t-Bu)-Ser(t-Bu)-Gly-OHRelated products of tetrahydrofuran

You may like
-
 Selenium dichloride oxide CAS 7791-23-3View Details
Selenium dichloride oxide CAS 7791-23-3View Details
7791-23-3 -
 2-Fluoro-6-iodobenzoic acid 111771-08-5 98+View Details
2-Fluoro-6-iodobenzoic acid 111771-08-5 98+View Details
111771-08-5 -
![3-(4-bromo-3-methyl-2-oxo-2,3-dihydro-1H-benzo[d]imidazol-1-yl)piperidine-2,6-dione 98+](https://img.chemicalbook.in//Content/image/CP5.jpg) 3-(4-bromo-3-methyl-2-oxo-2,3-dihydro-1H-benzo[d]imidazol-1-yl)piperidine-2,6-dione 98+View Details
3-(4-bromo-3-methyl-2-oxo-2,3-dihydro-1H-benzo[d]imidazol-1-yl)piperidine-2,6-dione 98+View Details
2304754-51-4 -
 2,3-Difluoro-6-methoxybenzyl Chloride 1073435-67-2 98+View Details
2,3-Difluoro-6-methoxybenzyl Chloride 1073435-67-2 98+View Details
1073435-67-2 -
 2-(6-(benzyloxy)-3,4-dihydronaphthalen-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane 98+View Details
2-(6-(benzyloxy)-3,4-dihydronaphthalen-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane 98+View Details
2477812-43-2 -
 KT-474 98+View Details
KT-474 98+View Details
2432994-31-3 -
 7646-85-7 Zinc Chloride Solution (All Grades) As requiredView Details
7646-85-7 Zinc Chloride Solution (All Grades) As requiredView Details
7646-85-7 -
 Radiator Flux As requiredView Details
Radiator Flux As requiredView Details
