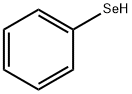
Phenylselenenyl chloride
Synonym(s):Benzeneselenenyl chloride;Benzeneselenyl chloride
- CAS NO.:5707-04-0
- Empirical Formula: C6H5ClSe
- Molecular Weight: 191.52
- MDL number: MFCD00000478
- EINECS: 227-196-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
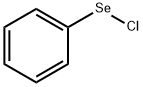
What is Phenylselenenyl chloride?
Chemical properties
crystalline solid
The Uses of Phenylselenenyl chloride
Phenylselenenyl chloride is used in the preparation of (cyclobut-1-enylselanyl)benzene. It is an important versatile reagent used in organic synthesis. Further, it serves as a useful synthon for 2-amino alcohols.
What are the applications of Application
Phenylselenyl chloride is a versatile synthesis reagent
General Description
Phenylselenyl chloride is a synthon for 2-amino alcohols. It is a versatile reagent in organic synthesis.
Purification Methods
Purify it by distillation in a vacuum, and recrystallisation (orange needles) from hexane [Foster J Am Chem Soc 55 822 1933, Foster et al. Recl Trav Chim, Pays-Bas 53 405, 408 1934, Behaghel & Seibert Chem Ber 66 714 1933]. [Beilstein 6 III 1110.] HIGHLY TOXIC.
Properties of Phenylselenenyl chloride
| Melting point: | 59-62 °C (lit.) |
| Boiling point: | 120 °C/20 mmHg (lit.) |
| Flash point: | 120°C/20mm |
| storage temp. | Store below +30°C. |
| solubility | Soluble in methanol. |
| form | crystal |
| color | red |
| Sensitive | Moisture Sensitive |
| Hydrolytic Sensitivity | 7: reacts slowly with moisture/water |
| BRN | 1237091 |
| Exposure limits | ACGIH: TWA 0.2 mg/m3 NIOSH: IDLH 1 mg/m3; TWA 0.2 mg/m3 |
| Stability: | Stable, but may be air or moisture sensitive. Incompatible with strong bases, strong oxidizing agents. |
| InChI | InChI=1S/C6H5ClSe/c7-8-6-4-2-1-3-5-6/h1-5H |
| CAS DataBase Reference | 5707-04-0(CAS DataBase Reference) |
Safety information for Phenylselenenyl chloride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H290:Corrosive to Metals H373:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P234:Keep only in original container. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P314:Get medical advice/attention if you feel unwell. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Phenylselenenyl chloride
| InChIKey | WJCXADMLESSGRI-UHFFFAOYSA-N |
| SMILES | C1([Se]Cl)=CC=CC=C1 |
Phenylselenenyl chloride manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Phenylselenenyl Chloride CAS 5707-04-0View Details
Phenylselenenyl Chloride CAS 5707-04-0View Details
5707-04-0 -
 Phenylselenyl chloride CAS 5707-04-0View Details
Phenylselenyl chloride CAS 5707-04-0View Details
5707-04-0 -
 Phenylselenyl chloride Cas No 5707-04-0View Details
Phenylselenyl chloride Cas No 5707-04-0View Details
5707-04-0 -
 Phenyl Selenyl ChlorideView Details
Phenyl Selenyl ChlorideView Details
5707-04-0 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
Statement: All products displayed on this website are only used for non medical purposes such as industrial applications or scientific research, and cannot be used for clinical diagnosis or treatment of humans or animals. They are not medicinal or edible.
