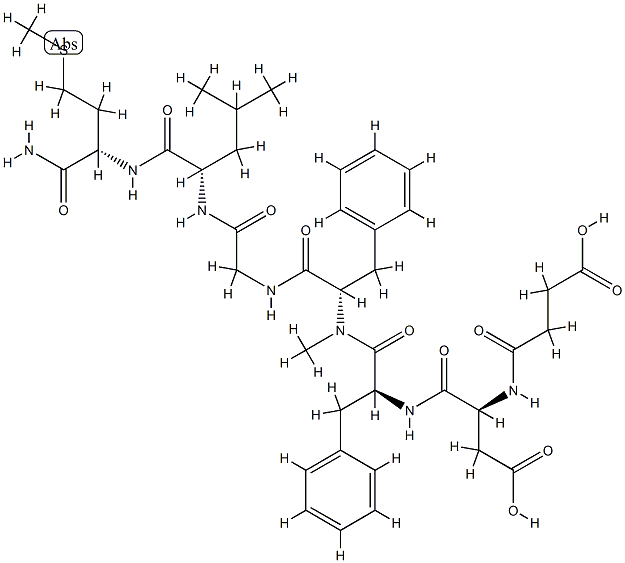
Secukinumab
- CAS NO.:875356-43-7
- Molecular Weight: 0
- Update Date: 2024-11-19 20:33:22
What is Secukinumab?
Description
Secukinumab is currently the first and only fully humanized biological agent that can specifically inhibit interleukin 17A (IL-17A). IL-17A is a core pathogenic factor involved in inflammation and disease progression in psoriasis (PsO), psoriasis arthropathy (PsA) and ankylosing spondylitis (AS), and plays a cornerstone role in the pathogenesis.
The Uses of Secukinumab
Secukinumab is a human interleukin-IL-17A antagonist indicated for the treatment of adults with moderate to severe plaque psoriasis as an alternative to systemic therapy or phototherapy.
Mechanism of action
Secukinumab is unique from other approved therapies for psoriasis as it is a first-in-class recombinant high-affinity, fully human monoclonal antibody of the IgG1/kappa isotype that selectively targets IL-17A. Secukinumab interferes in the psoriasis pathologic process by selectively binding to IL-17A, thereby preventing IL-17A interaction with the IL-17 receptor expressed on, for example, keratinocytes[1].
Pharmacokinetics
Secukinumab's pharmacokinetics (PK) profile is typical of a fully human immunoglobulin IgG1 molecule: Slow subcutaneous (SC) absorption, low clearance, and long half-life. Clearance of secukinumab in geriatric patients and patients <65 years of age was similar. Because secukinumab is a human IgG with a large molecular size (~150 kDa) and because intact immunoglobulin is filtered by the kidney to a minimal degree, only very small amounts of antibody are expected to be excreted in the urine. Thus, renal impairment is not likely to influence urinary excretion and the overall PK profile. Finally, hepatic impairment would not be expected to influence the metabolism or excretion of human IgG. Secukinumab's main route of elimination is through intracellular catabolism. Thus, the potential for drug interactions between secukinumab and small drug molecules is low.
Side Effects
The most common adverse reactions (>1%) with secukinumab were nasopharyngitis, diarrhea, and upper respiratory tract infection.
References
[1] Godse, Kiran. “Secukinumab - First in Class Interleukin-17A Inhibitor for the Treatment of Psoriasis.” Indian Journal of Dermatology 62 2 (2017): 195–199.
Safety information for Secukinumab
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran






You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8