Immunoglobulin G4,anti-(human integrin R4) (human-mouse monoclonal AN100226 c4-chain),disulfide with human-mouse monoclonal AN100226 light chain,dimer
- CAS NO.:189261-10-7
- Empirical Formula: C40H55N7O11S
- Molecular Weight: 841.97
- Update Date: 2024-07-02 08:54:58
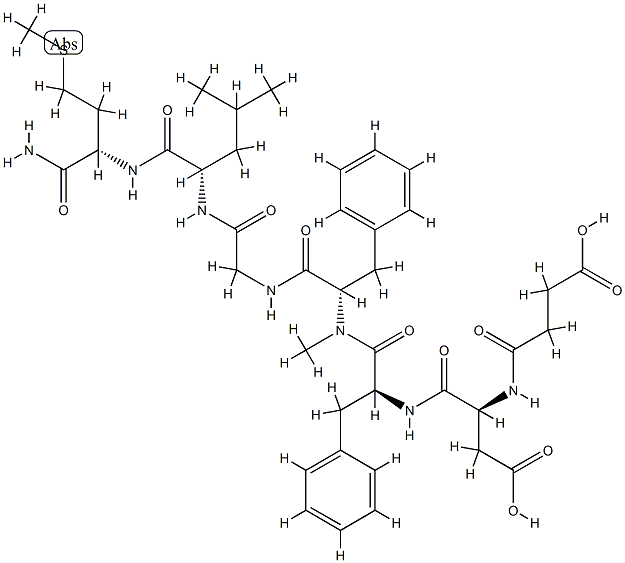
What is Immunoglobulin G4,anti-(human integrin R4) (human-mouse monoclonal AN100226 c4-chain),disulfide with human-mouse monoclonal AN100226 light chain,dimer?
Description
The a4 family of integrins expressed on the surface of leukocytes are involved in cell adhesion processes. The α4 integrin can pair with either of two b subunits to generate a heterodimeric cell surface receptor known as α4β1 (VLA4) or α4β7. Ligands for VLA4 include vascular cell adhesion molecule-1 (VCAM-1), which is expressed on activated vascular endothelium, while α4β7 interacts predominantly with mucosal addressin cell adhesion molecule-1 (MadCAM-1) existing on vascular endothelial cells of the gastrointestinal tract. By virtue of this α4–mediated interaction between leukocytes and vascular endothelial cells that leads to trans-endothelial infiltration of various leukocytes (lymphocytes, monocytes, T-cells, etc.) at the site of inflammation, interference with the adhesion of the α4 integrin has been deemed a viable approach for disrupting the inflammatory cascade. As an antibody that binds to the α4 integrin subunit, natalizumab has been developed and launched for the treatment of multiple sclerosis, a chronic inflammatory disorder of the central nervous system. It is also being developed for other chronic inflammatory diseases, such as, Crohn’s disease, rheumatoid arthritis, and irritable bowel syndrome (IBS). Natalizumab is a recombinant humanized monoclonal antibody produced in murine myeloma cells. It contains human framework regions and the complementarity-determining regions of an antibody that is targeted to the α4 integrin. For the treatment of irritable bowel diseases (Crohn’s disease, ulcerative colitis, and IBS), the target is the α4β7 glycoprotein while efficacy in treating MS is attributed to binding to the α4 subunit of α4β1. For MS, the binding of natalizumab prevents docking of VCAM-1 to its receptor on leukocytes, thereby, effectively inhibiting leukocyte trafficking across the blood brain-barrier (BBB). A reduction in migration across the BBB translates into a reduction in lesions and relapses. In a two-year, placebo-controlled, double blind phase III study, a oncemonthly, 300 mg i.v. infusion of natalizumab reduced relapses by 66% compared to placebo. All of the secondary endpoints, such as, the number of new or newly enlarging T2-hyperintense lesions, the number of gadolinium-enhancing lesions, and the proportion of relapse-free patients, were all met. Regarding side effects, headache, fatigue, and arthralgia were reported in 5% of natalizumab patients, 2% more common than observed with placebo. Serious hypersensitivity-like reactions were experienced in 1% of the natalizumab group. In these cases, adverse effects usually developed within two hours of the onset of the infusion. The symptoms included urticaria, fever, rash, rigors, dizziness, pruritus, nausea, flushing, dyspnea, hypotension, and chest pain. Antibodies to natalizumab are believed to be responsible, and any patient experiencing hypersensitivity should discontinue further treatment. Since adequate studies have not been performed in the pregnant, pediatric, and elderly, natalizumab is currently contraindicated in these patient populations. In addition, this drug should not be taken concurrent with medications that suppress the immune system, such as, corticosteroids; the combination increases the risk for serious infections. With a dose of 300 mg to MS patients, the long half-life of 11±4 days results in a once-a-month trip to the physician for the one-hour infusion. Natalizumab is cleared at a rate of 16 mL/h with a Cmax of 98 μg/ mL and a mean steady-state concentration of approximately 30 μg/mL.
Originator
Elan (UK)
brand name
Tysabri
Properties of Immunoglobulin G4,anti-(human integrin R4) (human-mouse monoclonal AN100226 c4-chain),disulfide with human-mouse monoclonal AN100226 light chain,dimer
| storage temp. | Store at 4°C, do not freeze |
| form | Liquid |
| color | Colorless to light yellow |
Safety information for Immunoglobulin G4,anti-(human integrin R4) (human-mouse monoclonal AN100226 c4-chain),disulfide with human-mouse monoclonal AN100226 light chain,dimer
Computed Descriptors for Immunoglobulin G4,anti-(human integrin R4) (human-mouse monoclonal AN100226 c4-chain),disulfide with human-mouse monoclonal AN100226 light chain,dimer
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran







You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1