Salinomycin
Synonym(s):Salinomycin
- CAS NO.:53003-10-4
- Empirical Formula: C42H70O11
- Molecular Weight: 751
- MDL number: MFCD25541652
- EINECS: 258-290-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-27 17:31:30
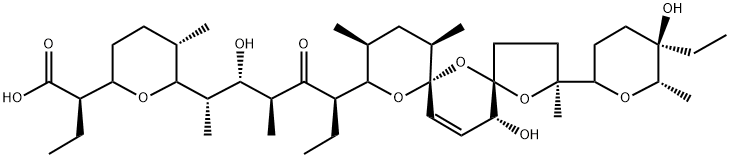
What is Salinomycin?
Description
Salinomycin was found in the culture mycelium of Streptomyces albus by Otake et al. of the University of Tokyo in 1973. Like other polyether ionophore antibiotics, it shows activity against gram-positive bacteria, fungi, and Coccidium. Salinomycin has been used as a feed additive to protect poultry against coccidial infections.
Chemical properties
solid
The Uses of Salinomycin
Salinomycin was used as a standard in the development of LC-MS/MS method for detection of residual coccidiostats in egg and muscle samples. It was used to screen out CD44+CD24- mesenchymal-like subpopulation within breast carcinomas.
The Uses of Salinomycin
antibacterial
The Uses of Salinomycin
Salinomycin is a polyether ionophore with broad spectrum Gram positive and anti-coccidial activity. Salinomycin has a high affinity for monovalent cations, particularly potassium. Salinomycin is used to control coccidia in animals and for growth promotion in ruminants. Recently, salinomycin has been shown to inhibit cancer stem cells and is >100 times more potent than taxol. While the mechanism of action is unknown, it was noted that among the 60,000 compounds screened, another monovalent ionophore, nigericin, and a chloride channel inhibitor, avermectin, were also active.
What are the applications of Application
Salinomycin is a coccidiostat and carboxylic polyether potassium ionophore with antibiotic and anti-cancer properties.
Definition
ChEBI: Salinomycin is a polyketide and a spiroketal. It has a role as an animal growth promotant and a potassium ionophore.
General Description
Chemical structure: polyether
Biological Activity
salinomycin (sal), which is a polyether ionophore antibiotic from streptomyces albus, has been proven to be able to kill different types of human cancer cells, most likely via interfering with abc drug transporters, the wnt/β-catenin signaling pathway, or other pathways.
Biochem/physiol Actions
Salinomycin produced by Streptomyces albus is a carboxylic polyether ionophore with antibiotic and anti-cancer properties. It induces cell death in some types of cancer cells such as breast, lung, gastric cancer, leukemia and osteosarcoma. Salinomycin inhibits multidrug resistance protein 1 and induces apoptosis by the generation of reactive oxygen species that cause DNA damage and inactivation of Stat3. Use of salinomycin as antibiotic results in lowered spermatozoa count and motility and decreased steroidogenesis in mice.
in vitro
several hepatocellular carcinoma (hcc) cell lines were treated with sal. results showed that sal inhibited proliferation and decreased pcna levels. cell cycle analysis showed that sal caused cell cycle arrest in different phases. sal induced apoptosis as characterized by an increase in the bax/bcl-2 ratio. compared to control, β-catenin expression was down-regulated by sal treatment significantly. the ca2+ concentration in hcc cells was examined by flow cytometry and it was found that higher ca2+ concentrations were observed in sal treatment groups [1].
in vivo
the in vivo anti-tumor effect of sal was verified using the hepatoma orthotopic tumor model and results showed that the liver tumor size in sal-treated groups decreased. immunohistochemistry and tunel staining also demonstrated that sal could in vivo inhibit proliferation and induced apoptosis [1].
References
[1] wang f,he l,dai wq,xu yp,wu d,lin cl,wu sm,cheng p,zhang y,shen m,wang cf,lu j,zhou yq,xu xf,xu l,guo cy. salinomycin inhibits proliferation and induces apoptosis of human hepatocellular carcinoma cells in vitro and in vivo. plos one.2012;7(12):e50638.
Properties of Salinomycin
| Melting point: | 112.5-113.5 °C(lit.) |
| Boiling point: | 839.2±65.0 °C(Predicted) |
| alpha | D25 -63° (c = 1 in ethanol) |
| Density | 1.18±0.1 g/cm3(Predicted) |
| Flash point: | >110°(230°F) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | insoluble in H2O; ≥142.2 mg/mL in EtOH; ≥91.8 mg/mL in DMSO |
| form | White solid |
| pka | 6.4 (DMF) |
| color | White to light yellow |
| Water Solubility | Soluble in methanol. Insoluble in water |
| Merck | 13,8415 |
| Stability: | Stable, but may be heat sensitive - keep cool. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 53003-10-4(CAS DataBase Reference) |
Safety information for Salinomycin
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H300:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Salinomycin
Salinomycin manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 Salinomycin 98.00% CAS 53003-10-4View Details
Salinomycin 98.00% CAS 53003-10-4View Details
53003-10-4 -
 Salinomycin CAS 53003-10-4View Details
Salinomycin CAS 53003-10-4View Details
53003-10-4 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1