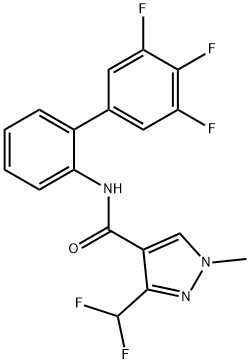
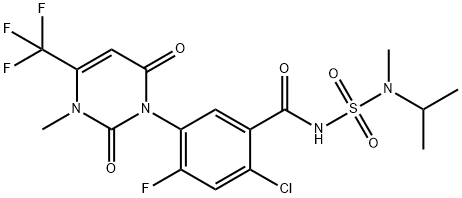
Saflufenacil
Synonym(s):N′-{2-Chloro-4-fluoro-5-[1,2,3,6-tetrahydro-3-methyl-2,6-dioxo-4-(trifluoromethyl)pyrimidin-1-yl]benzoyl}-N-isopropyl-N-methylsulfamide;2-Chloro-5-[3,6-dihydro-3-methyl-2,6-dioxo-4-(trifluoromethyl)-1(2H)-pyrimidinyl]-4-fluoro-N-{[methyl(1-methylethyl)amino]sulfonyl}benzamide
- CAS NO.:372137-35-4
- Empirical Formula: C17H17ClF4N4O5S
- Molecular Weight: 500.85
- MDL number: MFCD16875523
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-27 17:31:30
What is Saflufenacil?
The Uses of Saflufenacil
Saflufenacil-d7 is the labeled version of Saflufenacil (S081800), which is a herbicide of the pyrimidinedione chemical class used for the preplant burndown and selective preemergence dicot weed control in different field crops. It was found to inhibit protoporphyrinogen IX oxidase (PPO) enzyme.
Definition
ChEBI: Saflufenacil is a member of the class of sulfamides that is sulfamide in which one of the amino groups has been substituted by a methyl group an an isopropyl group, while the other has been substituted by a 2-chloro-4-fluoro-5-[3-methyl-2,6-dioxo-4-(trifluoromethyl)-3,6-dihydropyrimidin-1(2H)-yl]benzoyl group. An important BASF herbicide, not registered in Europe but sold in USA, Canada and several other countries to control weeds in a wide range of food crops. Often mixed with other products such as glyphosate. It has a role as an EC 1.3.3.4 (protoporphyrinogen oxidase) inhibitor, a herbicide and an agrochemical. It is a member of monochlorobenzenes, a member of monofluorobenzenes and a member of sulfamides. It is functionally related to a uracil.
Mode of action
Physiological and biochemical profiling confirmed that saflufenacil was a protoporphyrinogen-IX-oxidase (PPO) inhibitor. Saflufenacil elicits symptoms similar to other peroxidizing herbicides, including rapid, light-dependent necrosis of foliar tissue. Saflufenacil is readily absorbed by the root and shoot tissue of plants. Once absorbed, Saflufenacil is predominantly translocated via the xylem, with limited movement via the phloem. Saflufenacil is highly effective in controlling dicot weeds through contact and residual activity. In addition to non-crop and pre-plant burndown utility, saflufenacil demonstrates preemergence selectivity in a wide range of crops. Selectivity is conferred by physical placement and rapid metabolism of saflufenacil in tolerant crop species such as corn.
Properties of Saflufenacil
| Density | 1.542±0.06 g/cm3(Predicted) |
| solubility | DMSO ()Slightly, Methanol (Slightly) |
| pka | 4.24±0.40(Predicted) |
| form | neat |
| BRN | 11326826 |
| InChI | InChI=1S/C17H17ClF4N4O5S/c1-8(2)25(4)32(30,31)23-15(28)9-5-12(11(19)6-10(9)18)26-14(27)7-13(17(20,21)22)24(3)16(26)29/h5-8H,1-4H3,(H,23,28) |
| EPA Substance Registry System | Benzamide, 2-chloro-5-[3,6-dihydro-3-methyl-2,6-dioxo-4-(trifluoromethyl)-1(2H)-pyrimidinyl]-4-fluoro-N-[[methyl(1-methylethyl)amino]sulfonyl]- (372137-35-4) |
Safety information for Saflufenacil
| Signal word | Warning |
| Pictogram(s) |
 Environment GHS09 |
| GHS Hazard Statements |
H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P391:Collect spillage. Hazardous to the aquatic environment P501:Dispose of contents/container to..… |
Computed Descriptors for Saflufenacil
| InChIKey | GNHDVXLWBQYPJE-UHFFFAOYSA-N |
| SMILES | C(NS(N(C)C(C)C)(=O)=O)(=O)C1=CC(N2C(=O)N(C)C(C(F)(F)F)=CC2=O)=C(F)C=C1Cl |
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium 3-Bromopyrazole (3aR,4R,5R,6aS)-hexahydro-5-Triethyl silyloxy-4-((E)-3-oxo-5-phenylpent-1- enyl)cyclopenta[b]furan-2-one. 1-Chlorocarbonyl-4-piperidinopiperidine 1-Bromo-4-phenyl-2-Butanone 4-Amino-2-fluoro-N-methylbenzamide 1,1'-Carbonyldiimidazole SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTIONRelated products of tetrahydrofuran
You may like
-
 Saflufenacil CAS 372137-35-4View Details
Saflufenacil CAS 372137-35-4View Details
372137-35-4 -
 Saflufenacil CAS 372137-35-4View Details
Saflufenacil CAS 372137-35-4View Details
372137-35-4 -
![Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%View Details
Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%View Details
54094-19-8 -
 85-81-4 99%View Details
85-81-4 99%View Details
85-81-4 -
![208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%View Details
208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%View Details
208111-98-2 -
 2033-24-1 99%View Details
2033-24-1 99%View Details
2033-24-1 -
 Meldrums acid 2033-24-1 99%View Details
Meldrums acid 2033-24-1 99%View Details
2033-24-1 -
 2-Aminopyridine 504-29-0 99%View Details
2-Aminopyridine 504-29-0 99%View Details
504-29-0