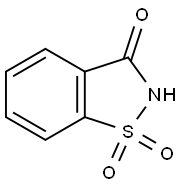
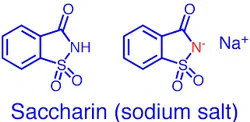
Saccharin
Synonym(s):o-Benzoic sulfimide;2,3-Dihydroxy-1,2-benzisothiazol-3-one-1,1-dioxide;2-Sulfobenzoic acid imide;Saccharin
- CAS NO.:81-07-2
- Empirical Formula: C7H5NO3S
- Molecular Weight: 183.18
- MDL number: MFCD00005866
- EINECS: 201-321-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:25
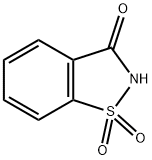
What is Saccharin?
Description
Saccharin was the first widely commercialized non-nutritive sweetener. It was discovered in 1879 by Constantin Fahlberg in the laboratory of Ira Remsen at Johns Hopkins University (Baltimore).
Fahlberg was working with the coal tar derivative benzoic sulfimide when he noticed a sweet taste on his hand.1 He connected the taste with the compound, which he gave the trivial name saccharin. He and Remsen soon developed a synthesis of saccharin from o-sulfamoylbenzoic acid. In the mid-1880s, Fahlberg, much to Remsen’s distress, began to manufacture saccharin in Germany.
As early as 1906, saccharin became controversial because of food additive concerns prompted by Upton Sinclair''s The Jungle. Harvey Wiley, the head chemist at the US Department of Agriculture, proposed a saccharin ban; but President Theodore Roosevelt, who needed to lose weight, declared, “Anyone who says saccharin is injurious to health is an idiot.” Wiley’s career was finished.
Saccharin’s use became widespread during World War I because of a sugar shortage. In the 1960s, it began to be promoted for weight loss, most familiarly under the trade name Sweet’n Low (Cumberland Packing Corp., Brooklyn).
Soon thereafter, food scientists discovered that saccharin causes bladder cancer in rats. In 1977 an act of Congress required the sweetener’s packaging to bear a cancer warning label. But in 2000, scientists found that humans metabolize saccharin differently from rats. The warning label requirement was rescinded.
Today, despite all the competition from other sweeteners and its metallic aftertaste, saccharin remains a popular choice.
1. In another version of the discovery, Fahlberg carelessly laid his cigarette on his lab bench. When he next smoked it, the cigarette tasted sweet.
Chemical properties
white crystalline solid
Chemical properties
Saccharin is a crystalline solid with a sweet taste (500 times sweeter than sugar).
Chemical properties
Saccharin occurs as odorless white crystals or a white crystalline powder. It has an intensely sweet taste, with a metallic or bitter aftertaste that at normal levels of use can be detected by approximately 25% of the population. The aftertaste can be masked by blending saccharin with other sweeteners.
History
Saccharin is the oldest and one of the best-known artificial sweeteners. It was accidentally discovered in 1878 by Ira Remsen (1846 1927) and his postdoctoral research fellow Constantin Fahlberg (1850 1910) at Johns Hopkins University when he was working on toluene derivatives from coal tar. He traced the taste back to the oxidized sulfonated chemicals he was working with and determined it was a sulfonated amide benzoic acid compound. Remsen and Fahlberg jointly published their findings on the compound in 1879 and 1880 in American and German journals. During the next several years, Remsen continued his academic work as one of the world's leading chemists, and Fahlberg perfected methods for commercialization of saccharin. Fours year after they published their work, Fahlberg and his uncle, Adolf List, applied for a United States patent for the compound, which was granted in 1885 (U.S. patent number 319082).
Saccharin was first introduced to the public in 1885. It was initially promoted as an antiseptic and food preservative. The use of saccharin as a sweetener started around 1900 when it was marketed for use by people with diabetes. Because saccharin
was a cheap sugar substitute, it was viewed as a threat to the sugar industry. Sugar manufacturers
in Europe, Canada, and the United States lobbied for laws restricting saccharin’s
use.
Calls to regulate saccharin in foods have been present throughout its history. Early in the 20th
century, the political climate promoted legislation and government oversight to ensure that food
was safe. In 1906, the passage of the Federal Food and Drug Act gave government regulatory
authority concerning the safety of food. The Department of Agriculture’s Bureau of Chemistry,
the predecessor of the Food and Drug Administration (FDA), performed research and made
recommendations with respect to food additives. In 1907, a study by the newly created Board
of Food and Drug Inspection made claims (latter refuted) that saccharin damaged the kidneys
and other organs. The leader of the Bureau of Chemistry, Harvey W. Wiley (1844–1930), was a
member of the Board and held the view that saccharin (and other chemicals such as benzoates)
was dangerous.
Approximately 30,000 tons per year of saccharin and saccharin salts are
used globally each year, with about 5,000 tons of this used in the United States.
Questions on saccharin’s safety has followed its usage to the present day. Saccharin is banned
in Canada (except in special cases), several European countries, and many other countries.
Countries where it is legal place restrictions on its use. Saccharin has been regulated in the
United States since the beginning of the century. A Canadian study in 1977 that reported saccharin
Legislation, signed into law on December 21, 2000, repealed the warning label requirement for products containing saccharin.
The National Cancer
Institute’s position is that there is no clear evidence linking saccharin to cancer in humans.
The Uses of Saccharin
It is a non-nutritive sweetener; pharmaceutic aid (flavor). Saccharin was formerly listed as reasonably anticipated to be a human carcinogen; delisted because the cancer data are not sufficient to meet the current criteria for this listing.
The Uses of Saccharin
Usually used in high performance liquid chromatographic method for the simultaneous separation and determination of acesulfame potassium, saccharine and aspartame;and also used in sweet preference test of rats.
The Uses of Saccharin
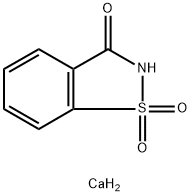
Saccharin is a non-nutritive synthetic sweetener which is 300–400 times sweeter than sucrose. it is nonhygroscopic and has a bitter aftertaste and a stability problem in cooked, canned, or baked goods. it is slightly soluble in water with a solubility of 10 g in 100 g of water at 25°c, but the solubility improves in boiling water. as sodium saccharin, there are two forms: 1,2-benzisothiazolin-3-one- 1,1-dioxide, sodium salt dihydrate, with a solubility of 1 g in 1.2 ml of water; and 1,2-benzisothiazolin-3-one-1,1-dioxide, sodium salt. calcium saccharin (chemical name: 1,2-benzisothiazolin-3-one-1, 1-dioxide, calcium salt) is used where low sodium content and reduced after-taste are required. it is used in low-calorie foods such as jam, beverages, and desserts. it is also termed sodium benzosulfimide.
What are the applications of Application
Saccharin is a non-nutritive sweetener
Definition
ChEBI: A 1,2-benzisothiazole having a keto-group at the 3-position and two oxo substituents at the 1-position. It is used as an artificial sweetening agent.
Production Methods
Saccharin is prepared from toluene by a series of reactions known as the Remsen–Fahlberg method. Toluene is first reacted with chlorosulfonic acid to form o-toluenesulfonyl chloride, which is reacted with ammonia to form the sulfonamide. The methyl group is then oxidized with dichromate, yielding o-sulfamoylbenzoic acid, which forms the cyclic imide saccharin when heated.
An alternative method involves a refined version of the Maumee process. Methyl anthranilate is initially diazotized to form 2- carbomethoxybenzenediazonium chloride; sulfonation followed by oxidation then yields 2-carbomethoxybenzenesulfonyl chloride. Amidation of this material, followed by acidification, forms insoluble acid saccharin.
Definition
A white crystalline organic compound used as an artificial sweetener; it is about 550 times as sweet as sugar (sucrose). It is nearly insoluble in water and so generally used in the form of its sodium salt. Possible links with cancer in animals has restricted its use in some countries.
Definition
saccharin: A white crystalline solid,C7H5NO3S, m.p. 224°C. It is madefrom a compound of toluene, derivedfrom petroleum or coal tar. It is awell-known artificial sweetener,being some 500 times as sweet assugar (sucrose), and is usually marketedas its sodium salt. Because ofan association with cancer in laboratoryanimals, its use is restricted insome countries.
Preparation
Saccharin is synthesized using two methods: the Remsen-Fahlberg process and the Maumee or Sherwin-Williams method. The Remsen-Fahlberg synthesis of saccharin starts by reacting toluene with chlorosulfonic acid to give ortho and para forms of toluene-sulfonic acid (Figure 78.1). The acid can be converted to sulfonyl chlorides by treating with phosphorus pentachloride. The ortho form, o-toluene-sulfonyl chloride, is treated with ammonia to give o-toluene-sulfonamide, which is then oxidized with potassium permanganate to produce o-sulfamido-benzoic acid. On heating, the latter yields saccharin. Another synthesis was developed at Maumee Chemical Company in Toledo, Ohio, and it came to be known as the Maumee process. This process starts with phthalic anhydride, which is converted into anthranilic acid. Anthranilic acid is then reacted with nitrous acid, sulfur dioxide, chlorine, and ammonia to give saccharin. The Maumee process was further refi ned by the Sherwin-Williams Company and is therefore now referred to as the Sherwin-Williams process.
brand name
Sweeta (Bristol-Myers Squibb).
General Description
White crystals. Odorless or faintly aromatic odor. Sweet taste.
Air & Water Reactions
Slightly soluble in water.
Reactivity Profile
An amide. Acid to litmus. pH of 0.35% aqueous solution: 2.0. Organic amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx).
Hazard
A questionable carcinogen. Products con- taining it must have a warning label.
Fire Hazard
Flash point data for Saccharin are not available; however, Saccharin is probably combustible.
Flammability and Explosibility
Non flammable
Pharmaceutical Applications
Saccharin is an intense sweetening agent used in beverages, food
products, table-top sweeteners, and oral hygiene products such as
toothpastes and mouthwashes. In oral pharmaceutical formulations,
it is used at a concentration of 0.02–0.5% w/w. It has been
used in chewable tablet formulations as a sweetening agent.
Saccharin has been used to form various pharmaceutical cocrystals.
Saccharin can be used to mask some unpleasant taste characteristics
or to enhance flavor systems. Its sweetening power is
approximately 300–600 times that of sucrose.
Biochem/physiol Actions
A sweet tastant for mammals. A glycerol taste receptor binding site specific for glucose has been proposed in drosophila.
Safety Profile
Confirmed carcinogen withexperimental neoplastigenic and tumorigenic data. Mildacute toxicity by ingestion. Experimental teratogenic andreproductive effects. Mutation data reported. Whenheated to decomposition it emits toxic NOx and SOx.
Safety
There has been considerable controversy concerning the safety of
saccharin, which has led to extensive studies since the mid-1970s.
Two-generation studies in rats exposed to diets containing
5.0–7.5% total saccharin (equivalent to 175 g daily in humans)
suggested that the incidence of bladder tumors was significantly
greater in saccharin-treated males of the second generation than in
controls. Further experiments in rats suggested that a contaminant
of commercial saccharin, o-toluene sulfonamide, might
also account for carcinogenic effects. In view of these studies, a ban
on the use of saccharin was proposed in several countries. However,
in 1977 a ban by the FDA led to a Congressional moratorium that
permitted the continued use of saccharin in the USA.
From the available data it now appears that the development of
tumors is a sex-, species-, and organ-specific phenomenon, and
extensive epidemiological studies have shown that saccharin intake
is not related to bladder cancer in humans.
The WHO has set a temporary acceptable daily intake for
saccharin, including its calcium, potassium, and sodium salts, at up
to 2.5 mg/kg body-weight. In the UK, the Committee on Toxicity
of Chemicals in Food, Consumer Products, and the Environment
(COT) has set an acceptable daily intake for saccharin and its
calcium, potassium, and sodium salts (expressed as saccharin
sodium) at up to 5 mg/kg body-weight.
Adverse reactions to saccharin, although relatively few in
relation to its widespread use, include: urticaria with pruritus
following ingestion of saccharin-sweetened beverages and
photosensitization reactions.
LD50 (mouse, oral): 17.5 g/kg
LD50 (rat, IP): 7.10 g/kg
LD50 (rat, oral): 14.2 g/kg
Potential Exposure
The information provided has to do, primarily, with the manufacturing of saccharin. Saccharin has been used as a nonnutritive sweetening agent. At one point the United States consumption pattern for all forms of saccharin has been estimated as 45% in soft drinks; 18% in tabletop sweeteners; 14% in fruits, juices, sweets, chew- ing gum, and jellies; 10% in cosmetics and oral hygiene products; 7% in drugs, such as coating on pills; 2% in tobacco; 2% in electroplating; and 2% for miscellaneous uses. Human exposure to saccharin occurs primarily through ingestion because of its use in many dietic foods and drinks and some personal hygiene products, including toothpastes and mouthwashes. The general public is exposed to saccharin, especially by persons required to reduce sugar intake.
Storage
Saccharin is stable under the normal range of conditions employed
in formulations. In the bulk form it shows no detectable
decomposition and only when it is exposed to a high temperature
(125°C) at a low pH (pH 2) for over 1 hour does significant
decomposition occur. The decomposition product formed is
(ammonium-o-sulfo)benzoic acid, which is not sweet. The
aqueous stability of saccharin is excellent.
Saccharin should be stored in a well-closed container in a dry
place.
Shipping
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous haz- ardous material, Technical Name Required.
Purification Methods
Purify saccharin by recrystallisation from Me2CO [solubility 7.14% at 0o, 14.4% at 50o], or aqueous isoPrOH to give a fluorescent solution. It sublimes in vacuo. It is an artificial sweetner and is 500 times sweeter than sucrose. [DeGarmo et al. J Am Pharm Assoc (Sci Ed) 41 17 1952, Beilstein 27 H 168, 870, 27 I 266, 27 II 214, 27 III/IV 2649.]
Incompatibilities
Dust may form explosive mixture with air. Incompatible with strong oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, and epoxides.
Incompatibilities
Saccharin can react with large molecules, resulting in a precipitate being formed. It does not undergo Maillard browning.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contami- nant (≥100 kg/mo) must conform to EPA regulations governing storage, transportation, treatment, and waste disposal.
Regulatory Status
Accepted for use as a food additive in Europe. Note that the EU number ‘E954’ is applied to both saccharin and saccharin salts. Included in the FDA Inactive Ingredients Database (oral solutions, syrups, tablets, and topical preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Properties of Saccharin
| Melting point: | 226-229 °C (lit.) |
| Boiling point: | subl |
| Density | 0.828 |
| vapor pressure | 0Pa at 25℃ |
| refractive index | 1.5500 (estimate) |
| storage temp. | Store below +30°C. |
| solubility | acetone: soluble1g in 12mL(lit.) |
| form | Crystals or Crystalline Powder |
| appearance | white crystals |
| pka | 11.68(at 18℃) |
| color | White |
| Odor | odorless |
| Water Solubility | 3.3 g/L (20 ºC) |
| Merck | 14,8311 |
| BRN | 6888 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 81-07-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Saccharin(81-07-2) |
| IARC | 3 (Vol. Sup 7, 73) 1999 |
| EPA Substance Registry System | Saccharin (81-07-2) |
Safety information for Saccharin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H351:Carcinogenicity H361:Reproductive toxicity |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Saccharin
| InChIKey | CVHZOJJKTDOEJC-UHFFFAOYSA-N |
Saccharin manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 81-07-2 Saccharin 98%View Details
81-07-2 Saccharin 98%View Details
81-07-2 -
 o-Sulfobenzimide CAS 81-07-2View Details
o-Sulfobenzimide CAS 81-07-2View Details
81-07-2 -
 Saccharine insoluble CAS 81-07-2View Details
Saccharine insoluble CAS 81-07-2View Details
81-07-2 -
 Saccharin 98% CAS 81-07-2View Details
Saccharin 98% CAS 81-07-2View Details
81-07-2 -
 SACCHARIN INSOLUBLE Extra Pure CAS 81-07-2View Details
SACCHARIN INSOLUBLE Extra Pure CAS 81-07-2View Details
81-07-2 -
 Saccharin ChemicalsView Details
Saccharin ChemicalsView Details
81-07-2 -
 Saccharin, For Food,Pharma SynthesisView Details
Saccharin, For Food,Pharma SynthesisView Details
81-07-2 -
 SaccharinView Details
SaccharinView Details
81-07-2
