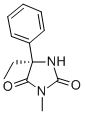
(S)-MEPHENYTOIN
Synonym(s):(±)-5-Ethyl-3-methyl-5-phenyl-2,4-imidazolidinedione;(±)-5-Ethyl-3-methyl-5-phenylhydantoin;(±)-Mephenytoin;(S)-(+)-5-Ethyl-3-methyl-5-phenyl-2,4-imidazolidinedione;(S)-(+)-5-Ethyl-3-methyl-5-phenylhydantoin
- CAS NO.:50-12-4
- Empirical Formula: C12H14N2O2
- Molecular Weight: 218.25
- MDL number: MFCD00270025
- EINECS: 200-012-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
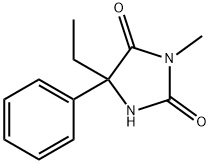
What is (S)-MEPHENYTOIN?
Description
Mephenytoin is N-methylated at position 3 with an ethyl group replacing one of the phenyl substituents at position 5. It is indicated for focal and jacksonian seizures in patients refractory to less toxic AEDs. Mephenytoin produces more sedation than phenytoin and should be used only when safer drugs have failed, because it is associated with an increased incidence of serious toxicities, such as severe rash, agranulocytosis, and hepatitis. Its N-desmethyl metabolite, 5-phenyl- 5-ethylhydantoin, contributes to both efficacy and toxicity for mephenytoin. The drug is no longer commercially available inside but is still available outside the United States.
Originator
Mesantoin,Sandoz
The Uses of (S)-MEPHENYTOIN
Anticonvulsant.
The Uses of (S)-MEPHENYTOIN
rac-Mephenytoin is an anticonvulsant agent.
Indications
For the treatment of refractory partial epilepsy.
Background
Mephenytoin is used for the treatment of refractory partial epilepsy. Mephenytoin is a solid. This compound belongs to the phenylhydantoins. These are heterocyclic aromatic compounds containing an imiazolidinedione moiety substituted by a phenyl group. Mephenytoin is known to target sodium channel protein type 5 subunit alpha. Cytochrome P450 2C19, Cytochrome P450 2C8, Cytochrome P450 2C9, Cytochrome P450 2B6, Cytochrome P450 1A2, and Cytochrome P450 2D6 are known to metabolize mephenytoin. Mephenytoin is a hydantoin-derivative anticonvulsant used to control various partial seizures. Mephenytoin and oxazolidinedione derivatives are associated with higher incidences of blood dyscrasias compared to other anticonvulsants.
Definition
ChEBI: Mephenytoin is an imidazolidine-2,4-dione (hydantoin) in which the imidazolidine nucleus carries a methyl group at N-3 and has ethyl and phenyl substituents at C-5. An anticonvulsant, it is no longer available in the USA or the UK but is still studied largely because of its interesting hydroxylation polymorphism. It has a role as an anticonvulsant.
Manufacturing Process
23 parts sodium was dissolved in 300 parts of ethanol and added to 160 parts of 5-phenylcyanacetamide in 750 parts of ethanol. A mixture was cooled straight away and a sodium salt of amide precipitated as a white powder. 200 parts of ethyl iodide was added to this mixture and heated for 1.5 hours. The ethanol was distilled off, water was added to the residue and rapidly hardened oil precipitated. After recrystallization from ethanol, 5-ethyl-5- phenylacetamide afforded; MP: 116°C.
100 parts of sodium hydroxide was solved in 500 parts of water and added to
83 parts of bromine by cooling. 5-Ethyl-5-phenylacetamide was added to
above prepared mixture. It dissolved quickly, whereupon all mass was heated
some time, cooled and stood at room temperature some hours. Then a
solution of sodium bisulfite was added before the formed precipitate dissolved.
The reaction mixture was filtered, the filtrate was acidified to give rapidly
hardened oil. After recrystallization from ethanol 5-ethyl-3-methyl-5-phenylhydantoin was yielded as the bright needles; MP: 201°-202°C.
brand name
Mesantoin (Novartis).
Therapeutic Function
Anticonvulsant, Antiepileptic
Biological Activity
CYP2C19 substrate. Anticonvulsant.
Biochem/physiol Actions
CYP2B6 and CYP2C19 substrate; anticonvulsive, antiepileptic.
Pharmacokinetics
Mephenytoin is an antiepileptic drug which can be useful in the treatment of epilepsy. The primary site of action appears to be the motor cortex where spread of seizure activity is inhibited. Possibly by promoting sodium efflux from neurons, mephenytoin tends to stabilize the threshold against hyperexcitability caused by excessive stimulation or environmental changes capable of reducing membrane sodium gradient. This includes the reduction of posttetanic potentiation at synapses. Loss of posttetanic potentiation prevents cortical seizure foci from detonating adjacent cortical areas. Mephenytoin reduces the maximal activity of brain stem centers responsible for the tonic phase of tonic-clonic (grand mal) seizures.
Side Effects
Side effects, including rash, fever, and fatal blood dyscrasia, prevent the use as an anticonvulsant drug of first choice.
Safety Profile
Poison by ingestion and intraperitoneal routes. Human systemic effects by ingestion: somnolence, hemorrhage, changes in teeth and supporting structures. Human mutation data reported. An experimental teratogen. An FDA proprietary drug used as an anticonvulsant. When heated to decomposition it emits toxic fumes of NOx.
Metabolism
Properties of (S)-MEPHENYTOIN
| Melting point: | 135-138 °C |
| Boiling point: | 358.94°C (rough estimate) |
| Density | 1.154±0.06 g/cm3 (20 ºC 760 Torr) |
| refractive index | 1.6660 (estimate) |
| storage temp. | 2-8°C |
| solubility | DMSO (Slightly), Ethanol (Slightly), Methanol (Slightly) |
| form | solid |
| pka | pKa 8.1 (Uncertain) |
| color | off-white |
| CAS DataBase Reference | 50-12-4(CAS DataBase Reference) |
| EPA Substance Registry System | Mephenytoin (50-12-4) |
Safety information for (S)-MEPHENYTOIN
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for (S)-MEPHENYTOIN
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran
![(2',5'-DIOXO-2,3-DIHYDRO-1'H-SPIRO[CHROMENE-4,4'-IMIDAZOLIDIN]-1'-YL)ACETIC ACID](https://img.chemicalbook.in/StructureFile/ChemBookStructure8/GIF/CB7193112.gif)
![(2,5-DIOXO-2',3'-DIHYDRO-1H-SPIRO[IMIDAZOLIDINE-4,1'-INDEN]-1-YL)ACETIC ACID](https://img.chemicalbook.in/StructureFile/ChemBookStructure8/GIF/CB6143291.gif)

![S-[4-14C]MEPHENYTOIN,S-[4-14C]MEPHENYTOIN](https://img.chemicalbook.in/StructureFile/ChemBookStructure4/GIF/CB2391469.gif)
You may like
-
 17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 -
 131987-69-4 98+View Details
131987-69-4 98+View Details
131987-69-4 -
 2-Hexyn-1-ol 98+View Details
2-Hexyn-1-ol 98+View Details
764-60-3 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 764-60-3 2-Hexyn-1-ol 98+View Details
764-60-3 2-Hexyn-1-ol 98+View Details
764-60-3 -
 2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
5968-21-8 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
