50-12-4
Product Name:
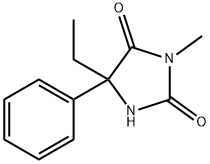
(S)-MEPHENYTOIN
Formula:
C12H14N2O2
Synonyms:
(±)-5-Ethyl-3-methyl-5-phenyl-2,4-imidazolidinedione;(±)-5-Ethyl-3-methyl-5-phenylhydantoin;(±)-Mephenytoin;(S)-(+)-5-Ethyl-3-methyl-5-phenyl-2,4-imidazolidinedione;(S)-(+)-5-Ethyl-3-methyl-5-phenylhydantoin
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Color/Form | Crystals |
| Melting Point | 135 °C |
| Solubility | 1270 mg/L |
| LogP | 1.69 |
| Decomposition | When heated to decomposition it emits toxic fumes of /nitrogen oxides/. |
| Dissociation Constants | 8.51 |
| Kovats Retention Index | 1795 1780 1786 1748 1765 1770 1791 1796 1796 |
| Other Experimental Properties | Forms a water-soluble sodium salt which has an alkaline reaction. |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 218.25 g/mol |
|---|---|
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 218.105527694 g/mol |
| Monoisotopic Mass | 218.105527694 g/mol |
| Topological Polar Surface Area | 49.4 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 310 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Mephenytoin is an imidazolidine-2,4-dione (hydantoin) in which the imidazolidine nucleus carries a methyl group at N-3 and has ethyl and phenyl substituents at C-5. An anticonvulsant, it is no longer available in the USA or the UK but is still studied largely because of its interesting hydroxylation polymorphism. It has a role as an anticonvulsant.
