(S)-(-)-LIMONENE
Synonym(s):(−)-p-Mentha-1,8-diene;(−)-Carvene;(S)-4-Isopropenyl-1-methyl cyclohexene
- CAS NO.:5989-54-8
- Empirical Formula: C10H16
- Molecular Weight: 136.23
- MDL number: MFCD00001558
- EINECS: 227-815-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
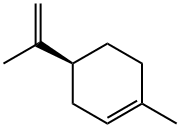
What is (S)-(-)-LIMONENE?
Description
ι-Limonene has a pleasant, lemon-like odor free from camphora ceous and turpentine-like notes. The most important and wide spread terpene; it is known in the d- and ι- optically active forms and in the optically inactive dl-form (known as dipentene); it has been reported found in more than 300 essential oils in amounts ranging from 90 - 95% (lemon, orange, mandarin) to as low as 1% (palmarosa);1 the most widespread form is the d-limonene, followed by the racemic form, and then ι-limonene.
Description
November is flavor and aroma month at MOTW!
Limonene is a monoterpene that exists in nature in two enantiomers: (S)-limonene [aka (–)-limonene, L-limonene] and (R)-limonene [aka (+)-limonene, D-limonene]. The (S)-isomer is shown here.
Both enantiomers have well-recognized flavors and aromas. (R)-Limonene1 is found in citrus oils and has the flavor and fragrance of oranges. (S)-Limonene is produced by coniferous trees and caraway, dill, and bergamot plants; its piny odor contributes to the flavors and aromas of the plants’ edible portions.
In addition to their commercial use as food flavorings, the limonenes are used in industrial cleaning solvents, wetting agents, air fresheners, and fragrances in personal care products. Worldwide production is ≈50,000 t/year with a 2020 market value of US$323 million.
Compounds like the limonenes were recently reported to have a downside. In a study conducted in 2018, Matthew Coggon and co-workers at the National Oceanic and Atmospheric Administration (Boulder, CO) collected air samples from various locations in New York City and analyzed the samples for volatile organic compounds (VOCs), which are notorious ozone generators.
The results, published in August 2021, showed that half of the VOCs were produced by fossil fuel combustion, an expected culprit for creating ozone. The other half came from chemical products, including solvents for coatings and adhesives, also typical ozone precursors. But fully half of the identified chemicals consisted largely of monoterpenes, such as limonene, and other substances used in personal care products. The study provides new clues for potential control of ozone formation in densely populated areas.
On the more positive side, (S)-limonene was in the news last month as an intermediate in an engineered biosynthetic process to make an unnatural terpene. John Hartwig and colleagues at the University of California, Berkeley, and Lawrence Berkeley National Laboratory created a strain of Escherichia coli to produce (S)-limonene, a Sulfolobus acidocaldarius2 enzyme, and a porphyrin-specific transporter protein inside its cells.
In a medium that contains an iridium mesoporphyrin and ethyl diazoacetate, the engineered E. coli imbibes the porphyrin, where it combines with the enzyme to promote an insertion reaction between the limonene and the diazo compound to form a cyclopropylterpene ester. The process mimics natural terpene-modification reactions with high stereoselectivity.
The limonenes should not be confused with limonin3, a bitter, highly complex citrus product, or lemonene, an obscure name for the aromatic compound biphenyl,4 which occurs naturally in coal tar and crude oil.
1. CAS Reg. No. 5989-27-5.
2. Sulfolobus acidocaldarius is a single-cell organism that grows at high temperature and acidity.
2. CAS Reg. No. 1180-71-8.
3. CAS Reg. No. 92-52-4.
Chemical properties
colourless liquid with lemon odour
Chemical properties
d-, l- or dl-Limonene has a pleasant, lemon-like odor free from camphoraceous and turpentine-like notes. Limonene is the most important and widespread terpene; it is known in the d- and l- optically active forms and in the optically inactive dl-form (known as dipentene).
Occurrence
It has been reported found in more than 300 essential oils in amounts ranging from 90 to 95% (lemon, orange, mandarin) to as low as 1% (palmarosa); the most widespread form is the d-limonene, followed by the racemic form and then l-limo nene. Also reported found in ginger, nutmeg, pepper, mace, hop oil, coriander seed, calamus, dill herb, caraway seed and rosemary.
The Uses of (S)-(-)-LIMONENE
(S)-(-)-Limonene is used to inhibit the proliferation of colon cancer cells. It is also used in artificial essential oils in order to produce (+)-carvone. It is an active component of turpentine. Further, it is used in flavorings, fragrances, and cosmetics. It finds application as a solvent and wetting agent. In addition to this it is used to produce resins.
What are the applications of Application
(S)-(?)-Limonene is which may inhibit the proliferation of colon cancer cells
Definition
ChEBI: An optically active form of limonene having (4S)-configuration.
Preparation
d-Limonene may be obtained by steam distillation of citrus peels and pulp resulting from the production of juice and cold pressed oils, or from deterpenation of citrus oils; it is sometimes redistilled.
Aroma threshold values
Detection: 4 to 229 ppb
Taste threshold values
Taste characteristics at 30 ppm: sweet, orange, citrus and terpy.
General Description
(S)-(-)-Limonene, a monoterpenoid compound, is mostly used in perfumery and in flavoring. On irradiation, it undergoes radiolysis, which leads to a reduction in the optical purity. (S)-(-)-Limonene also shows potent antimicrobial activity.
Flammability and Explosibility
Flammable
Contact allergens
Limonene is a racemic form of dand l-limonene. d-Limonene is contained in Citrus species such as citrus, orange, mandarin, and bergamot. l-Limonene is contained in Pinus pinea.
Safety Profile
A skin irritant. When heated to decomposition it emits acrid smoke and irritating fumes.
Properties of (S)-(-)-LIMONENE
| Melting point: | -74°C |
| Boiling point: | 175-177 °C(lit.) |
| alpha | -99.5 º (C=NEAT) |
| Density | 0.844 g/mL at 25 °C(lit.) |
| vapor density | 4.7 (vs air) |
| vapor pressure | <3 mm Hg ( 14.4 °C) |
| refractive index | n |
| Flash point: | 119 °F |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | 14 mg/l |
| solubility | Chloroform (Sparingly), Ethanol (Slightly), Methanol (Slightly) |
| appearance | colorless to pale yellow liquid |
| form | Liquid |
| color | Clear colorless to pale yellow |
| Odor | at 100.00 %. terpene pine herbal peppery |
| explosive limit | 0.7-6.1%(V) |
| optical activity | [α]20/D 94±4°, c = 10% in ethanol |
| Water Solubility | Insoluble |
| Merck | 14,5493 |
| BRN | 2323991 |
| Stability: | Stable. Incompatible with strong oxidizing agents. Flammable. |
| CAS DataBase Reference | 5989-54-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Cyclohexene, 1-methyl-4-(1-methylethenyl)-, (S)-(5989-54-8) |
| EPA Substance Registry System | Cyclohexene, 1-methyl-4-(1-methylethenyl)-, (4S)- (5989-54-8) |
Safety information for (S)-(-)-LIMONENE
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H226:Flammable liquids H304:Aspiration hazard H317:Sensitisation, Skin H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for (S)-(-)-LIMONENE
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran




You may like
-
 (S)-(-)-Limonene 99%View Details
(S)-(-)-Limonene 99%View Details -
 5989-54-8 99%View Details
5989-54-8 99%View Details
5989-54-8 -
 5989-54-8 98%View Details
5989-54-8 98%View Details
5989-54-8 -
 5989-54-8 99%View Details
5989-54-8 99%View Details
5989-54-8 -
 (-)-Limonene CAS 5989-54-8View Details
(-)-Limonene CAS 5989-54-8View Details
5989-54-8 -
 (S)-(-)-Limonene CAS 5989-54-8View Details
(S)-(-)-Limonene CAS 5989-54-8View Details
5989-54-8 -
 (S)-(−)-Limonene CAS 5989-54-8View Details
(S)-(−)-Limonene CAS 5989-54-8View Details
5989-54-8 -
 (S)-(−)-Limonene CAS 5989-54-8View Details
(S)-(−)-Limonene CAS 5989-54-8View Details
5989-54-8
