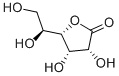
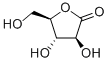
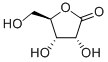
(S)-3-Hydroxy-gamma-butyrolactone
Synonym(s):(S)-4,5-Dihydro-4-hydroxy-2(3H)-furanone
- CAS NO.:7331-52-4
- Empirical Formula: C4H6O3
- Molecular Weight: 102.09
- MDL number: MFCD00211247
- EINECS: 434-990-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-09-24 16:40:18
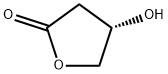
What is (S)-3-Hydroxy-gamma-butyrolactone?
Description
(S)-3-Hydroxy-gamma-butyrolactone is an important intermediate in organic synthesis and an important chiral pool. It is mainly used in the synthesis of some natural products and some bioactive chiral drugs or antibiotic chiral drugs. For example, it is a key intermediate in synthesis of nerve regulator (R)-GABOB and brain metabolic accelerant S-oxiracetam (S-ORC). It can be deoxidized to (S)-(+)-3-Hydroxytetrahydrofuran which is an important intermediate of anti-AIDS drugs. It is also used in the synthesis of S(-)-3-hydroxy-4-bromobutyric acid which is a potential stabilizer.
Chemical properties
Colorless to light yellow liquid.
The Uses of (S)-3-Hydroxy-gamma-butyrolactone
(S)-3-Hydroxy-γ-butyrolactone can be used as an anticancer drug resistance inhibitor.
What are the applications of Application
(S)-3-Hydroxy-gamma-butyrolactone is an important synthetic intermediate for a variety of chiral compounds. It is a key intermediate for preparing neuromediator (R)-GABOB, l-carnitine, and HMG-CoA reductase inhibitor, CI-981. (S)-3-Hydroxy-gamma-butyrolactonef has been reported as a satiety and potentiating agent to neuroleptic drugs. It is widely used in the pharmaceutical industry as a chiral building block for the statin class of cholesterol-reducing drugs such as Crestor and Lipitor, as well as the antibiotic Zyvox and the antihyperlipidemic medication Zetia. Other pharmaceuticals derived from 3HBL include HIV inhibitors and the nutritional supplement L-carnitine. 3HBL can readily be transformed into a variety of three-carbon building blocks and has been listed as one of the top value-added chemicals from biomass by the US Department of Energy[1-2].
Synthesis
Optically pure (S)-3-hydroxy-gamma-butyrolactone, a crucial chiral building block in the pharmaceutical industry, was synthesized from L: -malic acid by combining selective hydrogenation and lipase-catalyzed hydrolysis. Lipase from Candida rugosa was found to be the most efficient enzyme for the hydrolysis of (S)-beta-benzoyloxy-gamma-butyrolactone[1].
Precautions
For best results, Store in cool, dry place in tightly closed containers, under inert gas and protected from moisture as this substance is moisture sensitive. (S)-3-Hydroxy-gamma-butyrolactone is incompatible with oxidizing agents. This chemical causes skin irritation and serious eye irritation.
References
[1] Pradeep Kumar. “A simple and practical approach to enantiomerically pure (S)-3-hydroxy-γ-butyrolactone: synthesis of (R)-4-cyano-3-hydroxybutyric acid ethyl ester.” Tetrahedron, asymmetry 16 16 (2005): Pages 2717-2721. [2] Sang-Hyun Lee, Hong-Sun Uh, Oh-Jin Park. “A chemoenzymatic approach to the synthesis of enantiomerically pure (S)-3-hydroxy-γ-butyrolactone.” Applied Microbiology and Biotechnology 79 3 (2008): 355–362.
Properties of (S)-3-Hydroxy-gamma-butyrolactone
| Boiling point: | 98-100 °C0.3 mm Hg(lit.) |
| Density | 1.241 g/mL at 25 °C(lit.) |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | Inert atmosphere,2-8°C |
| pka | 12.87±0.20(Predicted) |
| optical activity | [α]21/D 81°, c = 2 in ethanol |
| Water Solubility | Miscible with water, alcohol and other organic solvent. Immiscible with light petroleum. |
| BRN | 1280864 |
| InChI | InChI=1S/C4H6O3/c5-3-1-4(6)7-2-3/h3,5H,1-2H2/t3-/m0/s1 |
| CAS DataBase Reference | 7331-52-4(CAS DataBase Reference) |
Safety information for (S)-3-Hydroxy-gamma-butyrolactone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for (S)-3-Hydroxy-gamma-butyrolactone
| InChIKey | FUDDLSHBRSNCBV-VKHMYHEASA-N |
| SMILES | O1C[C@@H](O)CC1=O |
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
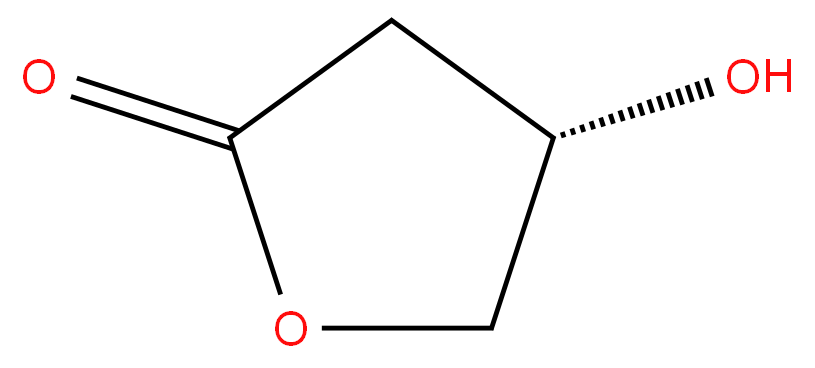 7331-52-4 (S)-4-Hydroxy dihydrofuran-2(3H)-one 85%View Details
7331-52-4 (S)-4-Hydroxy dihydrofuran-2(3H)-one 85%View Details
7331-52-4 -
 (S)-β-Hydroxy-γ-butyrolactone CAS 7331-52-4View Details
(S)-β-Hydroxy-γ-butyrolactone CAS 7331-52-4View Details
7331-52-4 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1