Rubrene
Synonym(s):5,6,11,12-Tetraphenylnaphthacene
- CAS NO.:517-51-1
- Empirical Formula: C42H28
- Molecular Weight: 532.67
- MDL number: MFCD00003703
- EINECS: 208-242-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-22 22:33:46
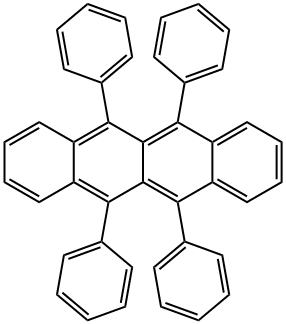
What is Rubrene?
Description
Rubrene, a molecule with a tetracene backbone and four appended phenyl rings, is one of the most studied molecular semiconductors due to its high charge mobility. Notably, room-temperature hole mobilities of the order of 20-40 cm2V-1s-1 have been measured for rubrene in single-crystal organic field-effect transistors (SC-OFET). It is widely used in organic electronics, especially organic light-emitting diodes (OLEDs) and organic field-effect transistors (OFETs).
Chemical properties
red crystalline powder
The Uses of Rubrene
Rubrene is an organic semiconductor used in organic light-emitting diodes (OLEDs) and organic field-effect transistors. It is used as a sensitizer in chemoluminesence. It is also used to prepare single crystal transistors. It acts as a reagent for chemiluminescence research and for transition metal complex ligation. Further, it is used as a p-type organic semiconductor.
What are the applications of Application
Rubrene is a reagent that is used in chemiluminescence research and transition metal complex ligation
Preparation
Rubrene is an organic molecule that has long been known for its outstanding semiconductor performance in organic electronic devices.
Rubrene is prepared by treating 1,1,3-triphenylprop-2-yne-1-ol with thionyl chloride.
The resulting chloroallene undergoes dimerization and dehydrochlorination to give rubrene.
General Description
Rubrene is a tetraphenyl derivative of tetracene that is used as an organic semiconductor. It is used as a source material in the fabrication of rubrene single crystal based transistors with carrier mobility over 10 cm2V?1s?1.
Purification Methods
It has also been recrystallised from *benzene under red light because it is chemiluminescent and light sensitive. [Beilstein 5 IV 2968.]
Structure and conformation
The rubrene molecule is basically the tetracene molecule with four wings. Its family are the polycyclic aromatic hydrocarbons. When rubrene molecules combine to build orthorhombic crystals, the molecules have a centrosymmetric structure with 2/m symmetry, as shown in the figure (their tetracene backbone acquires a twist when the molecules are free from constrains). Symmetry considerations imply that transitions between electronic ground state and first excited state can only be mediated by electromagnetic radiation that is polarized parallel to the 2-fold symmetry axis of the molecule (the M axis), which is in the plane of the tetracene backbone and perpendicular to its long axis.
https://www.lehigh.edu/~inlo/rubrene.html
Properties of Rubrene
| Melting point: | 330-335 °C (lit.) |
| Boiling point: | >315°C |
| Density | 1.1750 (estimate) |
| refractive index | 1.7160 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| form | powder |
| color | Red |
| Water Solubility | Soluble in hot toluene. Insoluble in water. |
| Sensitive | Air Sensitive |
| BRN | 1917339 |
| CAS DataBase Reference | 517-51-1(CAS DataBase Reference) |
| NIST Chemistry Reference | 5,6,11,12-Tetraphenylnaphthacene(517-51-1) |
| EPA Substance Registry System | Naphthacene, 5,6,11,12-tetraphenyl- (517-51-1) |
| Absorption | λmax?299 nm (in THF) |
Safety information for Rubrene
Computed Descriptors for Rubrene
| InChIKey | YYMBJDOZVAITBP-UHFFFAOYSA-N |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 517-51-1 Rubrene 98%View Details
517-51-1 Rubrene 98%View Details
517-51-1 -
 5,6,11,12-Tetraphenylnaphthacene CAS 517-51-1View Details
5,6,11,12-Tetraphenylnaphthacene CAS 517-51-1View Details
517-51-1 -
 Rubrene 98.00% CAS 517-51-1View Details
Rubrene 98.00% CAS 517-51-1View Details
517-51-1 -
 5,6,11,12-Tetraphenylnaphthacene (purified by sublimation) CAS 517-51-1View Details
5,6,11,12-Tetraphenylnaphthacene (purified by sublimation) CAS 517-51-1View Details
517-51-1 -
 Rubrene CAS 517-51-1View Details
Rubrene CAS 517-51-1View Details
517-51-1 -
 Rubrene CAS 517-51-1View Details
Rubrene CAS 517-51-1View Details
517-51-1 -
 Rubrene CAS 517-51-1View Details
Rubrene CAS 517-51-1View Details
517-51-1 -
 Rubrene CAS 517-51-1View Details
Rubrene CAS 517-51-1View Details
517-51-1
