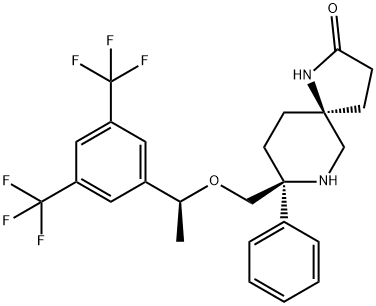
Rolapitant
- CAS NO.:552292-08-7
- Empirical Formula: C25H26F6N2O2
- Molecular Weight: 500.48
- MDL number: MFCD23105917
- EINECS: 812-147-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-22 11:16:47
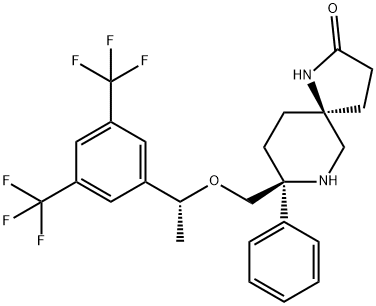
What is Rolapitant?
Absorption
Following administration of rolapitant, plasma concentrations reached peak levels in about 4 hours.
Toxicity
Most common adverse reactions occurring in >3% of patients receiving moderately emetogenic chemotherapy and combinations of anthracycline and cyclophosphamide included decreased appetite, neutropenia, dizziness, dyspepsia, urinary tract infection, stomatitis, and anemia. Most common adverse reactions occurring in patients receiving cisplatin-based highly emetogenic chemotherapy included neutropenia, hiccups, and abdominal pain.
Background
Rolapitant is a potent, highly selective, long-acting Neurokinin-1 (NK-1) receptor antagonist approved for the prevention of delayed chemotherapy-induced nausea and vomiting (CINV) in adults. Delayed-phase CINV typically occurs >24 hours after chemotherapy treatment and is principally mediated by Neurokinin-1 and its ligand Substance P, which is released in the gut following chemotherapy administration. Neurokinin-1 is also known as Tachykinin Receptor 1 (TACR1), Neurokinin 1 Receptor (NK1R), and Substance P Receptor (SPR). By blocking Substance P from interacting with NK-1 receptors in the gut and the central nervous system, rolapitant prevents late-phase CINV. Unlike other available NK-1 receptor antagonists, rolapitant is not an inhibitor of Cytochrome P450 enzyme CYP3A4 and has a long elimination half-life, allowing a single dose to prevent both acute and late-phase CINV during the first 120 hours post-chemotherapy.
Indications
This drug is indicated in adults in combination with other antiemetics for the prevention of delayed nausea and vomiting associated with emetogenic chemotherapy.
Definition
ChEBI: An azaspiro compound that is 1,7-diazaspiro[4.5]decan-2-one carrying additional phenyl and 1-{[3,5-bis(trifluoromethyl)phenyl]ethoxy}methyl substituents at position 8. Used (in the form of the hydrochloride hydrate) for the prevention of delayed nausea and vomiting associated with initial and repeat courses of emetogenic cancer chemotherapy.
Metabolism
Rolapitant is metabolized primarily by Cytochrome P450 enzyme 3A4 (CYP3A4) to its major active and circulating metabolite M19 (C4-pyrrolidine-hydroxylated rolapitant).
Properties of Rolapitant
| Boiling point: | 523.5±50.0 °C(Predicted) |
| Density | 1.34±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | DMF: 25 mg/ml; DMF:PBS(pH7.2) (1:2): 0.33 mg/ml; DMSO: 16 mg/ml; Ethanol: 3 mg/ml |
| form | Powder |
| pka | 15.88±0.40(Predicted) |
| color | White to off-white |
Safety information for Rolapitant
Computed Descriptors for Rolapitant
Rolapitant manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
