Riluzole
Synonym(s):2-Amino-6-(trifluoromethoxy)benzothiazole;2-Amino-6-(trifluoromethoxy)benzothiazole, PK 26124;Riluzole - CAS 1744-22-5 - Calbiochem
- CAS NO.:1744-22-5
- Empirical Formula: C8H5F3N2OS
- Molecular Weight: 234.2
- MDL number: MFCD00210213
- EINECS: 605-724-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 16:15:04
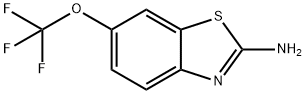
What is Riluzole?
Absorption
Riluzole is well-absorbed (approximately 90%), with average absolute oral bioavailability of about 60% (CV=30%). A high fat meal decreases absorption, reducing AUC by about 20% and peak blood levels by about 45%.
Description
Rilutek was launched in Germany, the UK and US (orphan drug status) for treatment of amyotrophic lateral sclerosis (ALS) and is the first drug approved for this indication. A one step synthesis from 4-(trifluoromethoxy)aniline provides a supply of the compound. The source of its neuroprotective and anticonvulsant activity is not clearly understood. It antagonizes excitatory amino acids and blocks presynaptic release of glutamate, is an antagonist of NMDA-induced acetylcholine release and inhibited glutamate and quisqualate induced increases in cGMP but does not bind to NMDA or Kainic receptors. Rilutek has no affinity for glutamate, GABAbenzodiazepine, glycine and adenosine receptors. It easily crosses the blood brain barrier and depresses glutamatergic neurotransmission, stabilizes voltagedependent Na channels in their inactive form and activates G-protein dependent processes.
Chemical properties
White Crystalline Solid
Originator
Rhone-Poulenc Rorer (France)
The Uses of Riluzole
Labeled Riluzole, intended for use as an internal standard for the quantification of Riluzole by GC- or LC-mass spectrometry.
The Uses of Riluzole
A neuroprotective agent. A glutamate release inhibitor. An anticonvulsant
The Uses of Riluzole
A neuroprotective agent. Modulates glutamatergic transmission. A glutamate release inhibitor. An anticonvulsant.
The Uses of Riluzole
anticonvulsant, glutamate release inhibitor, anti-ALS
What are the applications of Application
Riluzole is a sodium channel protein inhibitor
Background
A glutamate antagonist (receptors, glutamate) used as an anticonvulsant (anticonvulsants) and to prolong the survival of patients with amyotrophic lateral sclerosis. Riluzole is marketed as Rilutek by Sanofi.
Indications
For the treatment of amyotrophic lateral sclerosis (ALS, Lou Gehrig's Disease)
Definition
ChEBI: Riluzole is a member of benzothiazoles.
brand name
Rilutek (Sanofi Aventis).
Biological Activity
Novel psychotropic agent with anticonvulsant, hypnotic, anxiolytic, anti-ischemic and anesthetic properties. Riluzole is able to act as a glutamate release inhibitor, blocks voltage-dependent Na + channels and inhibits GABA uptake by striatal synaptosomes.
Biochem/physiol Actions
Glutamate release inhibitor; anticonvulsant
Pharmacokinetics
Riluzole, a member of the benzothiazole class, is indicated for the treatment of patients with amyotrophic lateral sclerosis (ALS). Riluzole extends survival and/or time to tracheostomy. It is also neuroprotective in various in vivo experimental models of neuronal injury involving excitotoxic mechanisms. The etiology and pathogenesis of amyotrophic lateral sclerosis (ALS) are not known, although a number of hypotheses have been advanced. One hypothesis is that motor neurons, made vulnerable through either genetic predisposition or environmental factors, are injured by glutamate. In some cases of familial ALS the enzyme superoxide dismutase has been found to be defective.
Metabolism
Riluzole is extensively metabolized to six major and a number of minor metabolites, which have not all been identified to date. Metabolism is mostly hepatic, consisting of cytochrome P450–dependent hydroxylation and glucuronidation. CYP1A2 is the primary isozyme involved in N-hydroxylation; CYP2D6, CYP2C19, CYP3A4, and CYP2E1 are considered unlikely to contribute significantly to riluzole metabolism in humans.
Storage
Room temperature
References
1) Bellingham (2011), A review of the neural mechanisms of action and clinical efficiency of riluzole in treating amyotrophic lateral sclerosis: what have we learned in the last decade; CNS Neurosci. Ther. 17 4 2) Urbani and Belluzzi (2000), Riluzole inhibits the persistent sodium current in mammalian CNS neurons; Eur. J. Neurosci. 12 3567 3) Frizzo et al. (2004), Riluzole enhances glutamate uptake in rat astrocyte cultures; Cell Mol. Neurobiol. 24 123 4) Doble (1996), The pharmacology and mechanism of action of riluzole; Neurology 47 S233 5) Pittenger et al. (2008), Riluzole in the treatment of mood and anxiety disorders; CNS Drugs 22 761 6) Namkoong et al. (2007), Metabotropic glutamate receptor 1 and glutamate signaling in human melanoma; Cancer Res. 67 2298 7) Zhang et al. (2015), Anti-cancer effect of metabotropic glutamate receptor inhibition in human glioma U87 cells: involvement of PI3K/Akt/mTOR pathway; Cell Physiol. Biochem. 35 419 8) Sperling et al. (2017), Riluzole: a potential therapeutic intervention in human brain tumor stem-like cells; Oncotarget 8 96697
Properties of Riluzole
| Melting point: | 116-118°C |
| Boiling point: | 296.3±50.0 °C(Predicted) |
| Density | 1.572±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: ≥25 mg/mL |
| form | solid |
| pka | 2.96±0.10(Predicted) |
| color | white |
| Merck | 14,8223 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 2 months. |
Safety information for Riluzole
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H300:Acute toxicity,oral |
Computed Descriptors for Riluzole
| InChIKey | FTALBRSUTCGOEG-UHFFFAOYSA-N |
Riluzole manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 Riluzole 98%View Details
Riluzole 98%View Details -
 1744-22-5 95-99 %View Details
1744-22-5 95-99 %View Details
1744-22-5 -
 Riluzole CAS 1744-22-5View Details
Riluzole CAS 1744-22-5View Details
1744-22-5 -
 Riluzole >98% (HPLC) CAS 1744-22-5View Details
Riluzole >98% (HPLC) CAS 1744-22-5View Details
1744-22-5 -
 Riluzole CAS 1744-22-5View Details
Riluzole CAS 1744-22-5View Details
1744-22-5 -
 Riluzole CAS 1744-22-5View Details
Riluzole CAS 1744-22-5View Details
1744-22-5 -
 Riluzole CAS 1744-22-5View Details
Riluzole CAS 1744-22-5View Details
1744-22-5 -
 Rilutek 50 Mg Tablet, Packaging Size: 3*10 TabletsView Details
Rilutek 50 Mg Tablet, Packaging Size: 3*10 TabletsView Details
1744-22-5
