RILPIVIRINE HCL
- CAS NO.:700361-47-3
- Empirical Formula: C22H19ClN6
- Molecular Weight: 402.89
- MDL number: MFCD11046523
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-07-02 08:55:13
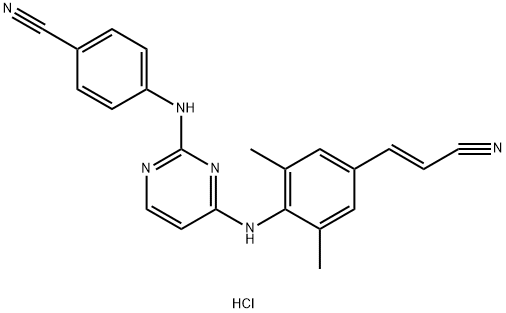
What is RILPIVIRINE HCL?
The Uses of RILPIVIRINE HCL
Rilpivirine Hydrochloride was shown to be able to treat and or prevent immunodeficiency virus-1. It also has uses for anti-viral therapy
Definition
ChEBI: A hydrochloride obtained by reaction of rilpivirine with one equivalent of hydrochloric acid. Used for treatment of HIV.
Clinical Use
Rilpivirine hydrochloride (Edurant), a non-nucleoside reverse transcriptase inhibitor (NNRTI), received its approval both from the U.S. FDA and E.U. EMA in 2011 for the treatment of HIV-1 infection in treatment-na?ve adult patients. It was discovered and developed by Janssen Pharmaceuticals and its subsidiary Tibotec Pharmaceuticals. As a second generation NNRTI, rilpivirine hydrochloride displayed higher potency and longer half-life with a 25 mg once a day dose, compared to existing NNRTIs, such as the 200 mg BID of efavirenze (Sustiva). In late 2011, the fixed-dose combination products of rilpivirine hydrochloride with two nucleoside reverse transcriptase inhibitor (RTIs) emtricitabine and tenofovir disoproxil fumarate, co-developed by Gilead Science and Tibotec, were also approved both by the FDA and EMA under brand names Complera® and Eviplera®, respectively.
Synthesis
Similar to efavirenze, rilpivirine hydrochloride is a diarylpyrimidine (DAPY) compound, and the large-scale process synthesis begins with commercially available 2-methylthio-4-pyrimidinone (193) shown in the scheme.
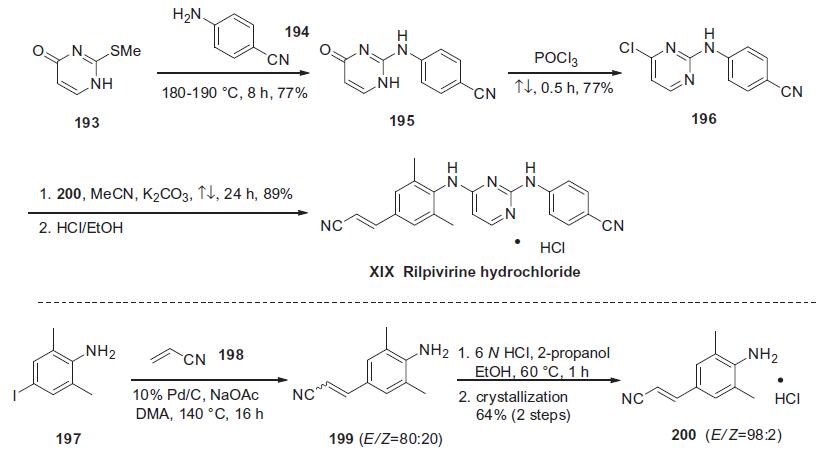
Thioether 193 was condensed with neat 4-cyanoaniline (194) at elevated temperature to afford diarylamine 195 in 77% yield. Subsequent treatment of pyrimidone 195 with refluxing POCl3 provided the corresponding chloride 196 in 77% yield.160,161 In the presence of K2CO3, chloride 196 was treated with the (E)-cinnamonitrile aniline 200 to give rilpivirine hydrochloride (XIX) in good yield.158 Aniline 200 was prepared via a Heck reaction of commercially available 4-iodo-2,6-dimethyl-benzeneamine (197) and acrylonitrile (198) affording compound 199 as a 4:1 mixture of E/Z isomers. The distribution of E/Z olefins was increased to 98:2 by salt formation and recrystallization to ultimately provide pure (E)-200 in 64% yield for two steps.
Properties of RILPIVIRINE HCL
| storage temp. | Store at -20°C |
| solubility | DMSO:50.0(Max Conc. mg/mL);136.46(Max Conc. mM) |
| form | Solid |
| color | White to off-white |
Safety information for RILPIVIRINE HCL
Computed Descriptors for RILPIVIRINE HCL
RILPIVIRINE HCL manufacturer
Arene Lifesciences Limited
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![4-[[4-[[4-[(E)-2-cyanoethenyl]-2,6-dimethyl-phenyl]amino]pyrimidin-2-yl]amino]benzonitrile](https://img.chemicalbook.in/CAS/GIF/500287-72-9.gif)
![DAPIVIRINE,4-[[4-(2,4,6-TRIMETHYLPHENYL)AMINO]PYRIMIDIN-2-YL]AMINO]BENZONITRILE](https://img.chemicalbook.in/CAS/GIF/244767-67-7.gif)

You may like
-
 700361-47-3 Rilpivirine hydrochloride 98%View Details
700361-47-3 Rilpivirine hydrochloride 98%View Details
700361-47-3 -
 700361-47-3 99%View Details
700361-47-3 99%View Details
700361-47-3 -
 Rilpivirine hydrochloride 99%View Details
Rilpivirine hydrochloride 99%View Details
700361-47-3 -
 700361-47-3 95-99%View Details
700361-47-3 95-99%View Details
700361-47-3 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1